CHEMICAL EQUATIONS Chemical equations You are expected to









- Slides: 26

CHEMICAL EQUATIONS

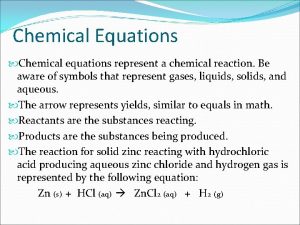
Chemical equations You are expected to know these names and formula: Name Formula Water H 2 O Hydrchloric Acid HCl Carbon Dioxide CO 2 Sulfuric Acid H 2 SO 4 Sulfur Dioxide SO 2 Nitric Acid HNO 3 Sulfur Trioxide SO 3 Ethanoic Acid CH 3 COOH Nitrogen Dioxide NO 2 Ammonia NH 4

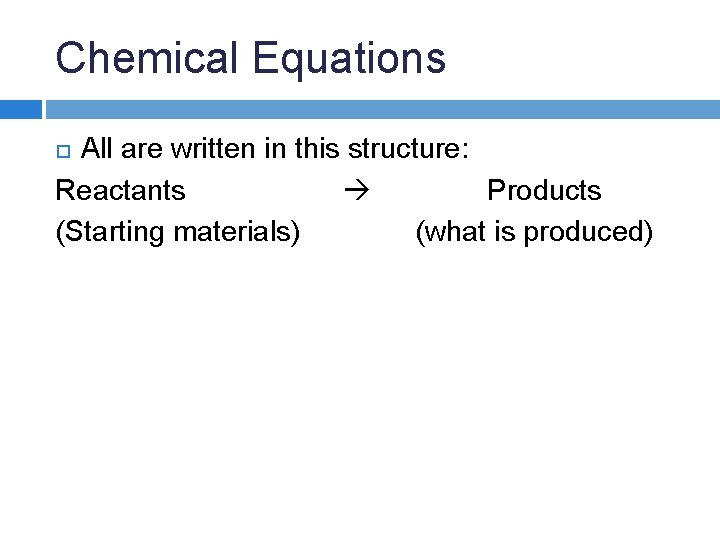
Chemical Equations All are written in this structure: Reactants Products (Starting materials) (what is produced)

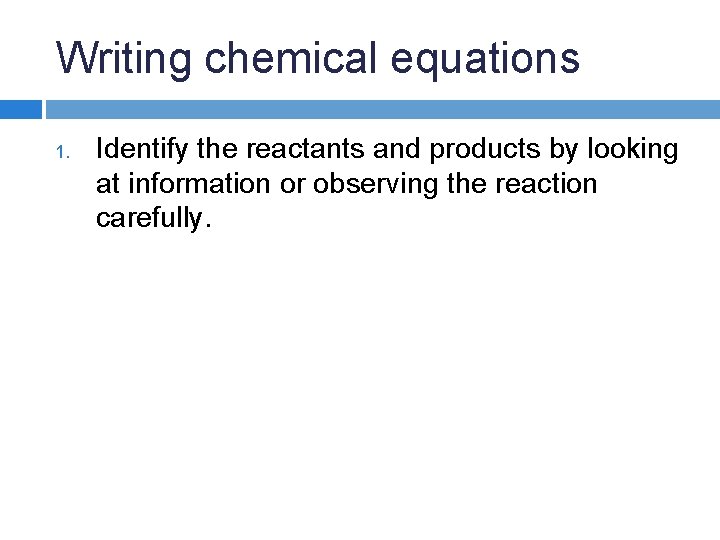
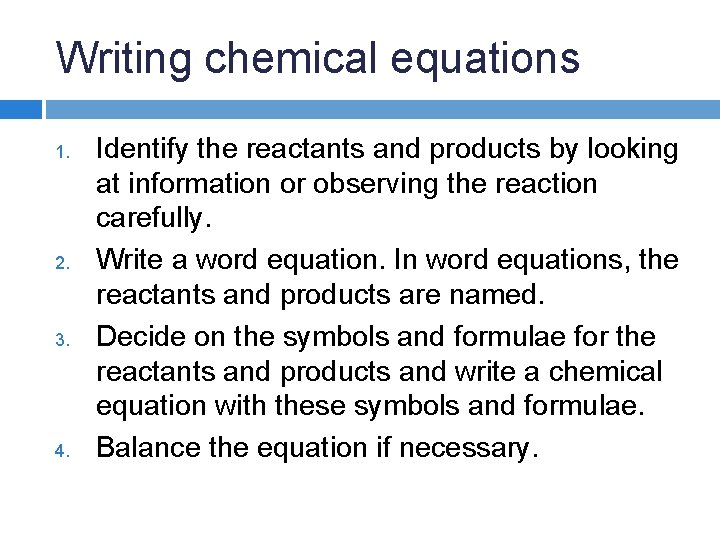
Writing chemical equations 1. Identify the reactants and products by looking at information or observing the reaction carefully.

Writing chemical equations 1. 2. Identify the reactants and products by looking at information or observing the reaction carefully. Write a word equation. In word equations, the reactants and products are named.

Writing chemical equations 1. 2. 3. Identify the reactants and products by looking at information or observing the reaction carefully. Write a word equation. In word equations, the reactants and products are named. Decide on the symbols and formulae for the reactants and products and write a chemical equation with these symbols and formulae.

Writing chemical equations 1. 2. 3. 4. Identify the reactants and products by looking at information or observing the reaction carefully. Write a word equation. In word equations, the reactants and products are named. Decide on the symbols and formulae for the reactants and products and write a chemical equation with these symbols and formulae. Balance the equation if necessary.

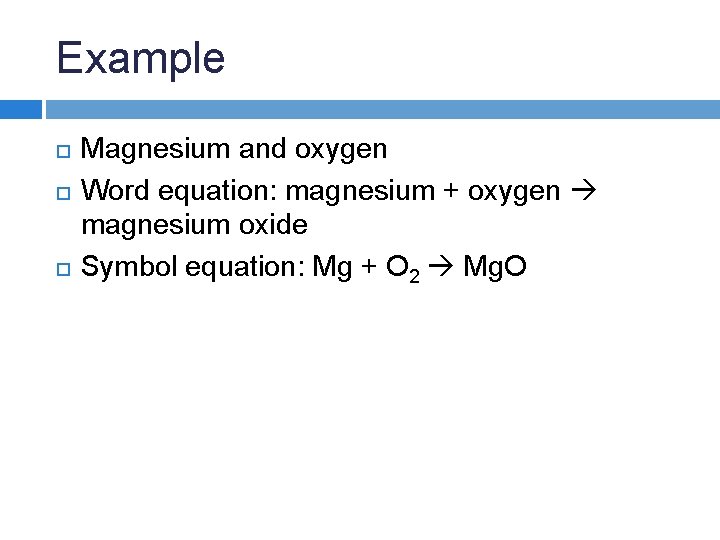
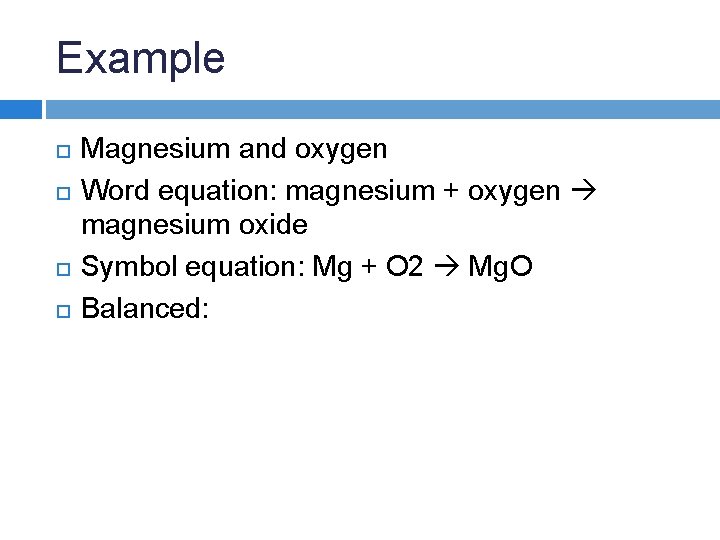
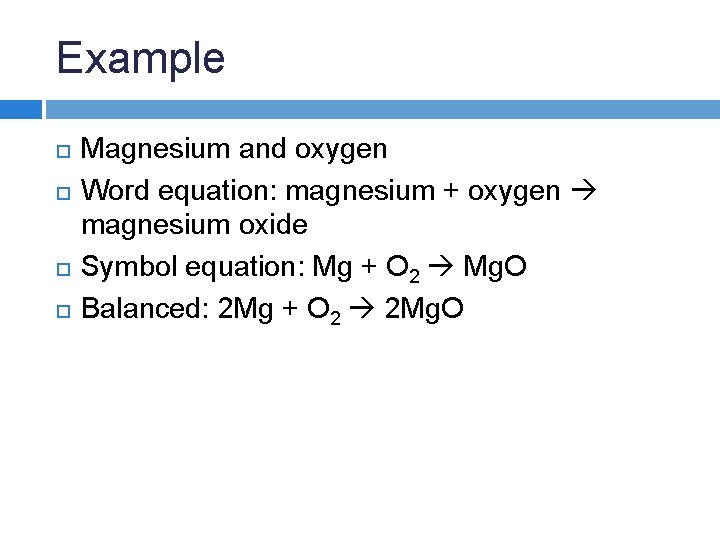
Example Magnesium and oxygen Word equation:

Example Magnesium and oxygen Word equation: magnesium + oxygen magnesium oxide Symbol equation:

Example Magnesium and oxygen Word equation: magnesium + oxygen magnesium oxide Symbol equation: Mg + O 2 Mg. O

Example Magnesium and oxygen Word equation: magnesium + oxygen magnesium oxide Symbol equation: Mg + O 2 Mg. O Balanced:

Example Magnesium and oxygen Word equation: magnesium + oxygen magnesium oxide Symbol equation: Mg + O 2 Mg. O Balanced: 2 Mg + O 2 2 Mg. O

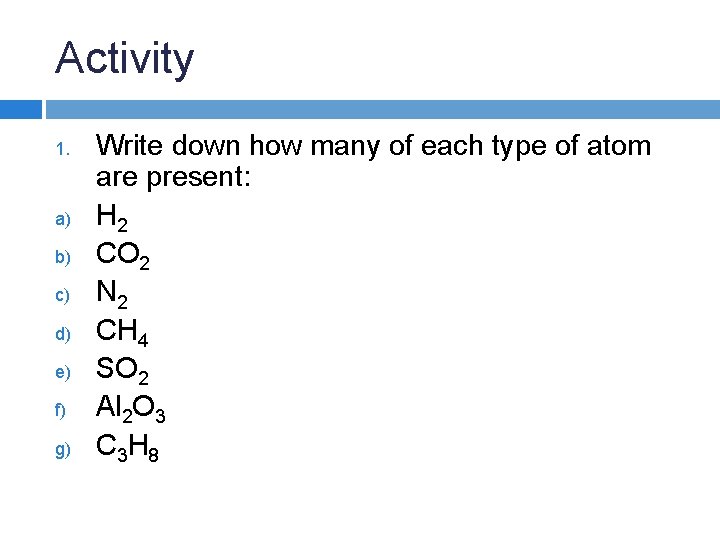
Activity 1. a) b) c) d) e) f) g) Write down how many of each type of atom are present: H 2 CO 2 N 2 CH 4 SO 2 Al 2 O 3 C 3 H 8

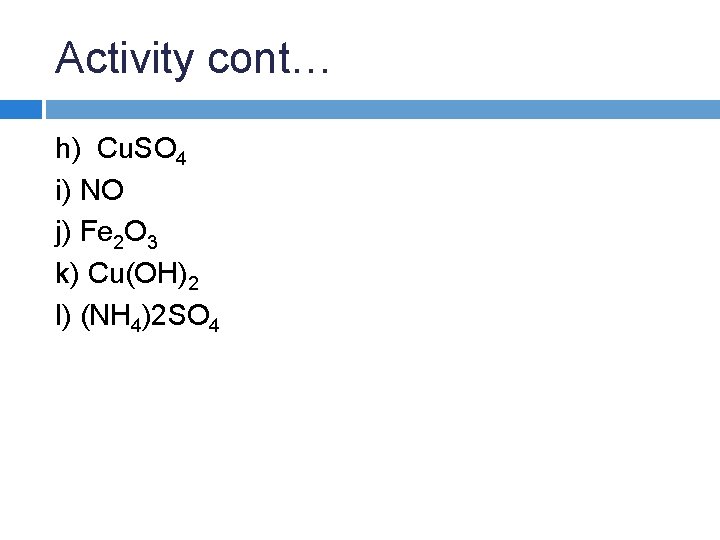
Activity cont… h) Cu. SO 4 i) NO j) Fe 2 O 3 k) Cu(OH)2 l) (NH 4)2 SO 4

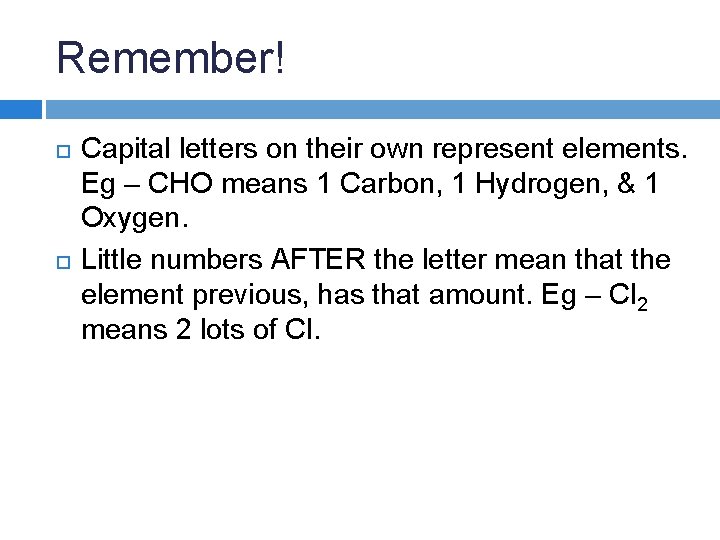
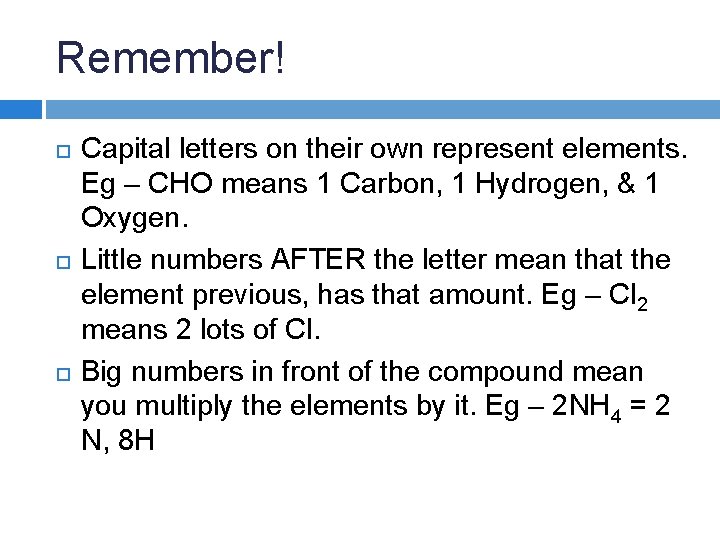
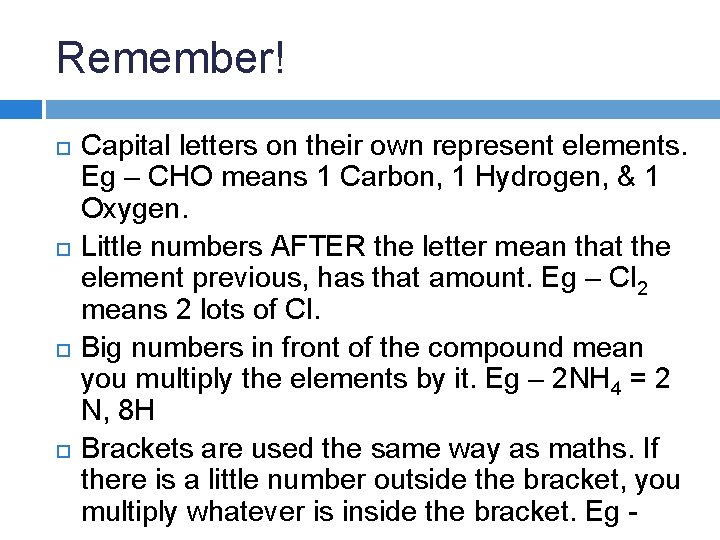
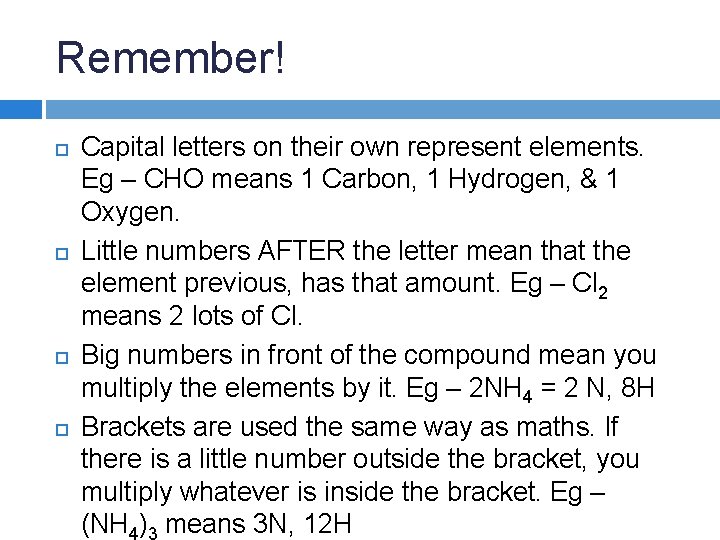
Remember! Capital letters on their own represent elements.

Remember! Capital letters on their own represent elements. Eg – CHO means 1 Carbon, 1 Hydrogen, & 1 Oxygen.

Remember! Capital letters on their own represent elements. Eg – CHO means 1 Carbon, 1 Hydrogen, & 1 Oxygen. Little numbers AFTER the letter mean that the element previous, has that amount. Eg – Cl 2 means 2 lots of Cl.

Remember! Capital letters on their own represent elements. Eg – CHO means 1 Carbon, 1 Hydrogen, & 1 Oxygen. Little numbers AFTER the letter mean that the element previous, has that amount. Eg – Cl 2 means 2 lots of Cl. Big numbers in front of the compound mean you multiply the elements by it. Eg – 2 NH 4 = 2 N, 8 H

Remember! Capital letters on their own represent elements. Eg – CHO means 1 Carbon, 1 Hydrogen, & 1 Oxygen. Little numbers AFTER the letter mean that the element previous, has that amount. Eg – Cl 2 means 2 lots of Cl. Big numbers in front of the compound mean you multiply the elements by it. Eg – 2 NH 4 = 2 N, 8 H Brackets are used the same way as maths. If there is a little number outside the bracket, you multiply whatever is inside the bracket. Eg -

Remember! Capital letters on their own represent elements. Eg – CHO means 1 Carbon, 1 Hydrogen, & 1 Oxygen. Little numbers AFTER the letter mean that the element previous, has that amount. Eg – Cl 2 means 2 lots of Cl. Big numbers in front of the compound mean you multiply the elements by it. Eg – 2 NH 4 = 2 N, 8 H Brackets are used the same way as maths. If there is a little number outside the bracket, you multiply whatever is inside the bracket. Eg – (NH ) means 3 N, 12 H

Answers a) b) c) d) e) f) g) h) i) j) k) l) 2 H 1 C, 2 O 2 N 1 C, 4 H 1 S, 2 O 2 Al, 3 O 3 C, 8 H 1 Cu, 1 S, 4 O 1 N, 1 O 2 Fe, 3 O 1 Cu, 2 O, 2 H 2 N, 8 H, S, 8 O

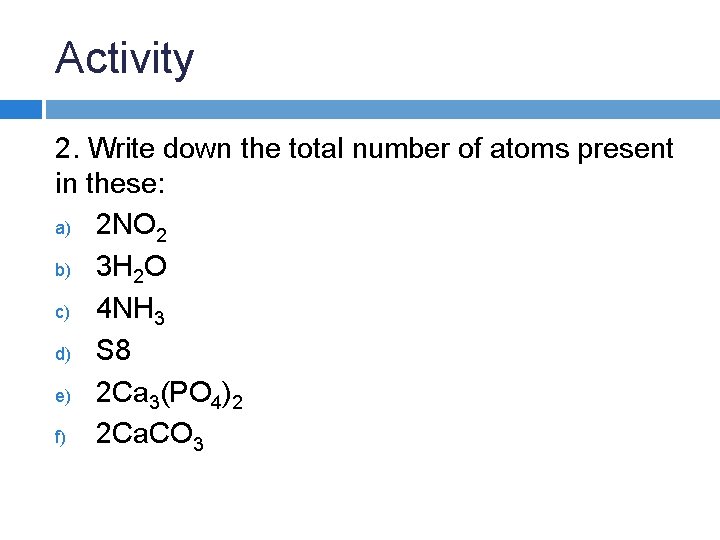
Activity 2. Write down the total number of atoms present in these: a) 2 NO 2 b) 3 H 2 O c) 4 NH 3 d) S 8 e) 2 Ca 3(PO 4)2 f) 2 Ca. CO 3

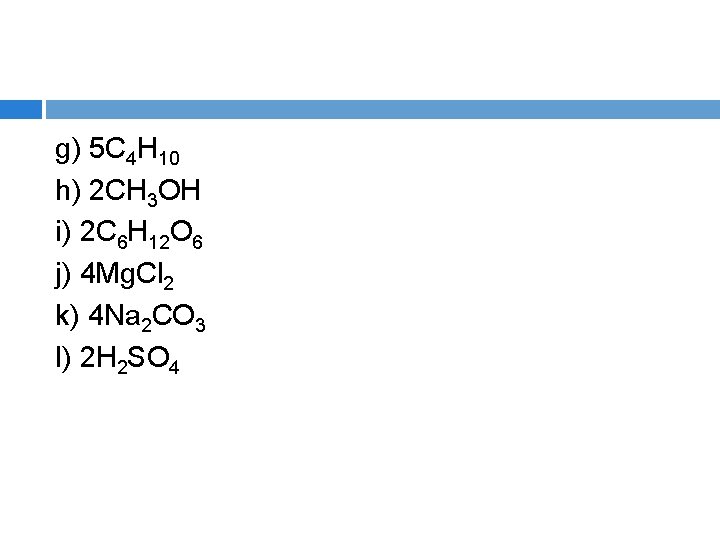
g) 5 C 4 H 10 h) 2 CH 3 OH i) 2 C 6 H 12 O 6 j) 4 Mg. Cl 2 k) 4 Na 2 CO 3 l) 2 H 2 SO 4

Answers a) b) c) d) e) f) g) h) i) j) k) l) 6 (2 N, 4 O) 9 (6 H, 3 O) 16 (4 N, 12 H) 8 (8 S) 26 (6 Ca, 4 P, 16 O) 10 (2 Ca, 2 C, 6 O) 70 (20 C, 50 H) 12 (2 C, 8 H, 2 O) 48 (12 C, 24 H, 12 O) 12 (4 Mg, 8 Cl) 24 (8 Na, 4 C, 12 O) 14 (4 H, 2 S, 8 O)

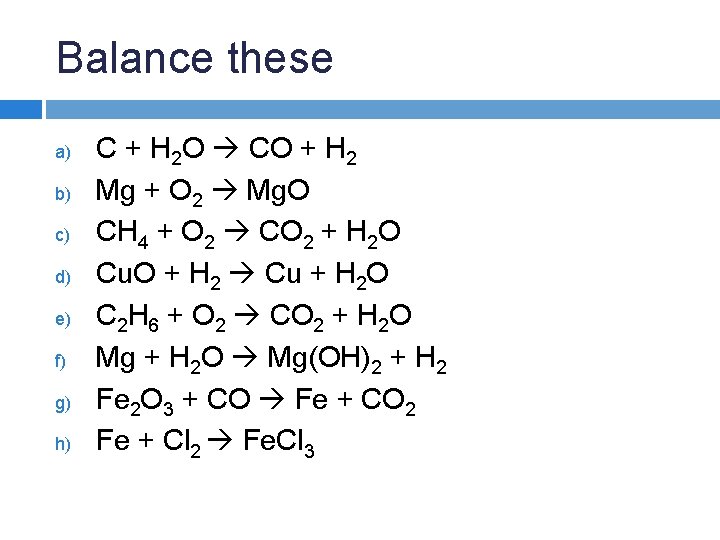
Balance these a) b) c) d) e) f) g) h) C + H 2 O CO + H 2 Mg + O 2 Mg. O CH 4 + O 2 CO 2 + H 2 O Cu. O + H 2 Cu + H 2 O C 2 H 6 + O 2 CO 2 + H 2 O Mg + H 2 O Mg(OH)2 + H 2 Fe 2 O 3 + CO Fe + CO 2 Fe + Cl 2 Fe. Cl 3

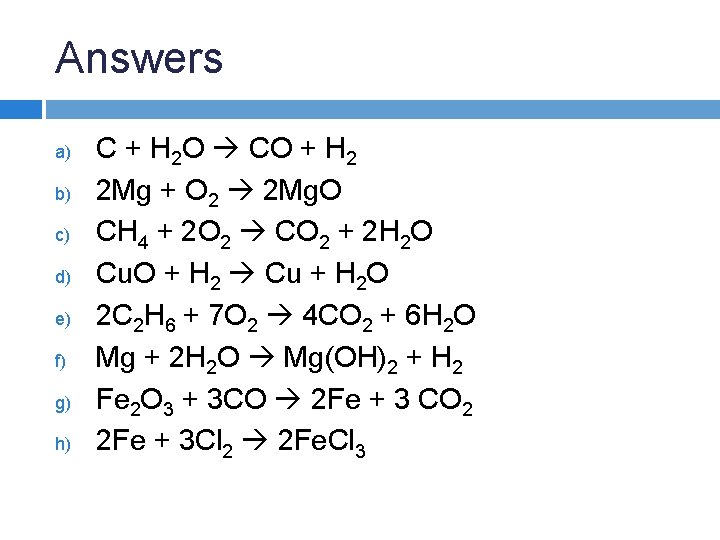
Answers a) b) c) d) e) f) g) h) C + H 2 O CO + H 2 2 Mg + O 2 2 Mg. O CH 4 + 2 O 2 CO 2 + 2 H 2 O Cu. O + H 2 Cu + H 2 O 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O Mg + 2 H 2 O Mg(OH)2 + H 2 Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 2 Fe + 3 Cl 2 2 Fe. Cl 3
 Insidan region jh
Insidan region jh Are kc and kp equal
Are kc and kp equal Translating chemical equations
Translating chemical equations You say you love the rain but you open your umbrella
You say you love the rain but you open your umbrella Eat meals that are nutritious agree or disagree
Eat meals that are nutritious agree or disagree If you think you can you can poem
If you think you can you can poem Tell me what you eat and i shall tell you what you are
Tell me what you eat and i shall tell you what you are I will follow you wherever
I will follow you wherever Introduction of work immersion
Introduction of work immersion Work immersion site
Work immersion site Water jet velocity formula
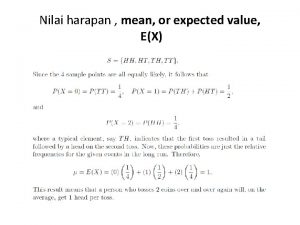
Water jet velocity formula Conditional expected value
Conditional expected value Value at risk formula
Value at risk formula Planning is a category of nursing behaviors in which: *
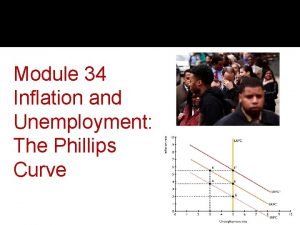
Planning is a category of nursing behaviors in which: * Long run phillips curve
Long run phillips curve Error expected identifier
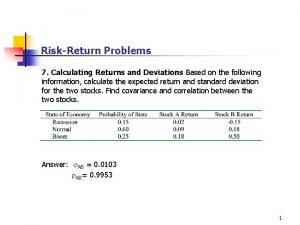
Error expected identifier Standard deviation of return
Standard deviation of return Portfolio variance
Portfolio variance Research proposal expected results example
Research proposal expected results example Regularized risk minimization
Regularized risk minimization 3 coin toss probability
3 coin toss probability Expected value probability
Expected value probability Nusing care plan
Nusing care plan Expected value adalah
Expected value adalah Short run phillips curve
Short run phillips curve Expected armored cavalry troop symbol
Expected armored cavalry troop symbol Lord kalki
Lord kalki