Characteristics of Solids When a solid changes to






- Slides: 15





Characteristics of Solids • When a solid changes to a liquid, the kinetic energies of the particles overcome the attractive forces holding them together. They break out of their crystalline structures. This kinetic energy needed corresponds to a definite melting point. • Since amorphous solids are much more unorganized, they do not have a definite melting point.

Supercooled Liquids • Amorphous solids are sometimes classified as supercooled liquids, which are substances that retain certain liquid properties even at temperatures at which they would normally be solid. • Supercooled Water

High Density and Incompressibility • In general, substances are most dense in the solid state (water is a counterexample of this) Solids tend to be slightly denser than liquids and much denser than gases. • Although it seems like solids such as a wine cork are sometimes compressible, they are not. The cork contains pores of air that are compressed when put under pressure. The solid matter in the cork itself is not.

Low Rate of Diffusion • If a zinc plate and copper plate are clamped together for a long time, eventually a few atoms will diffuse into the other. This observation shows that diffusion does occur in solids. The rate of diffusion is millions of times faster in liquids than in solids.

Crystal Structure • The total three-dimensional arrangement of particles of a crystal is called a crystal structure. – Think back to the marshmallows and toothpicks. You were building crystal structures when you combined them • The smallest portion of a crystal lattice that shows the three-dimensional pattern of the entire lattice is called a unit cell.

Review Questions Do not call out the answers - Choose one of the two underlined words 1. Crystalline solids are orderly/disorderly? 2. Solids have a definite/indefinite shape and volume 3. Amorphous solids do/do not have a set melting point 4. Metals diffuse slowly/not at all

Binding Forces in Crystal 1. Ionic Crystals: Positive and negative ions are arranged in a regular pattern. Usually formed when group 1 or 2 metals combine with group 16 or 17 nonmetals or nonmetallic polyatomic ions. - Strong binding forces between the ions make these structures hard, brittle, have a high melting point, and good insulators


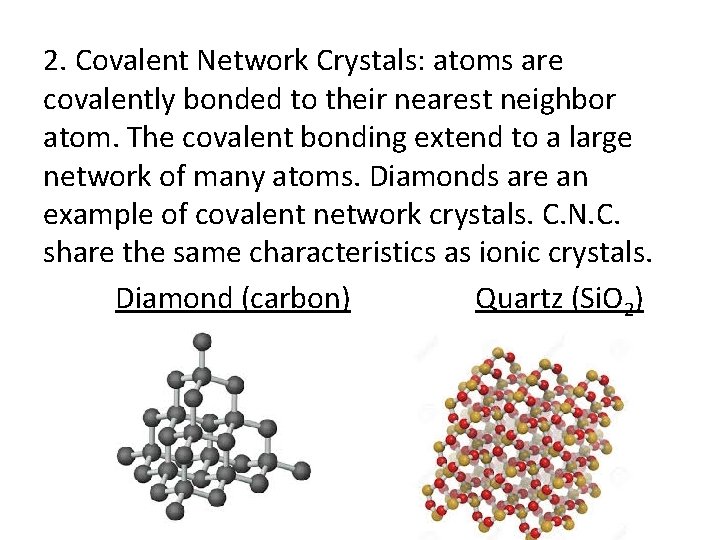
2. Covalent Network Crystals: atoms are covalently bonded to their nearest neighbor atom. The covalent bonding extend to a large network of many atoms. Diamonds are an example of covalent network crystals. C. N. C. share the same characteristics as ionic crystals. Diamond (carbon) Quartz (Si. O 2)

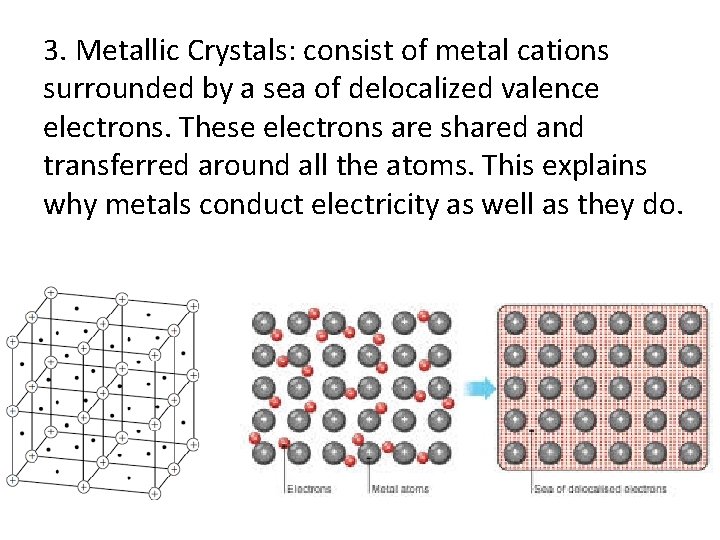
3. Metallic Crystals: consist of metal cations surrounded by a sea of delocalized valence electrons. These electrons are shared and transferred around all the atoms. This explains why metals conduct electricity as well as they do.

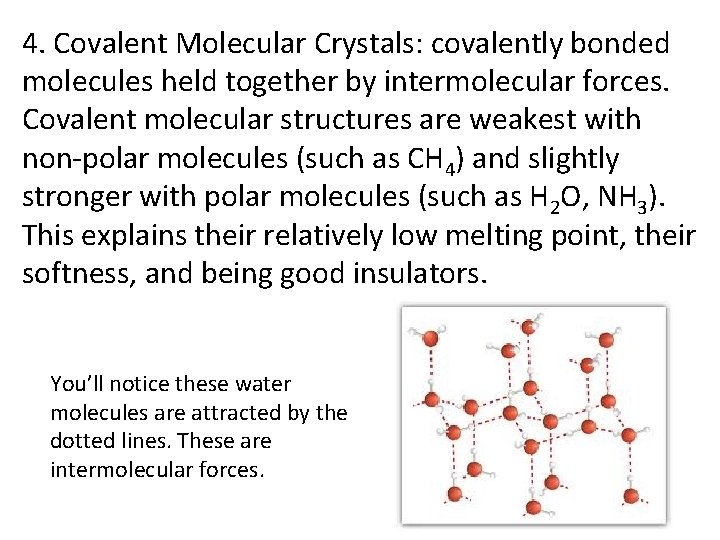
4. Covalent Molecular Crystals: covalently bonded molecules held together by intermolecular forces. Covalent molecular structures are weakest with non-polar molecules (such as CH 4) and slightly stronger with polar molecules (such as H 2 O, NH 3). This explains their relatively low melting point, their softness, and being good insulators. You’ll notice these water molecules are attracted by the dotted lines. These are intermolecular forces.