Chapter 7 Coenzymes and Vitamins Prentice Hall c

















- Slides: 45

Chapter 7 Coenzymes and Vitamins Prentice Hall c 2002 Chapter 7 1

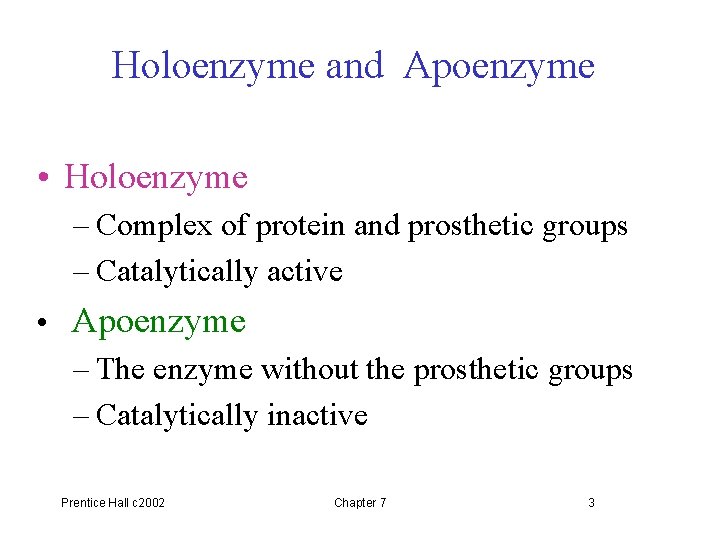
Coenzyme, p 192 -193 • Cofactors: nonprotein components • Cofactors may be metal ions or organic molecules (coenzyme) • Cofactor: metal ion + coenzyme • Prosthetic groups: tightly bound coenzymes Prentice Hall c 2002 Chapter 7 2

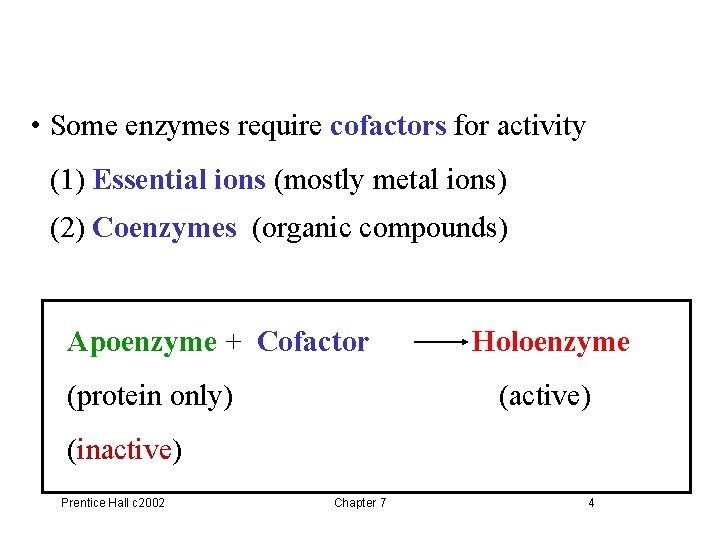
Holoenzyme and Apoenzyme • Holoenzyme – Complex of protein and prosthetic groups – Catalytically active • Apoenzyme – The enzyme without the prosthetic groups – Catalytically inactive Prentice Hall c 2002 Chapter 7 3

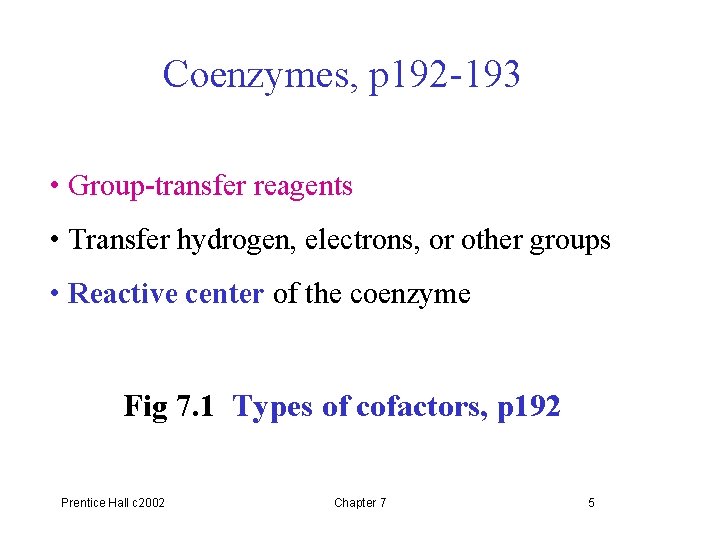
• Some enzymes require cofactors for activity (1) Essential ions (mostly metal ions) (2) Coenzymes (organic compounds) Apoenzyme + Cofactor (protein only) Holoenzyme (active) (inactive) Prentice Hall c 2002 Chapter 7 4

Coenzymes, p 192 -193 • Group-transfer reagents • Transfer hydrogen, electrons, or other groups • Reactive center of the coenzyme Fig 7. 1 Types of cofactors, p 192 Prentice Hall c 2002 Chapter 7 5

7. 1 Many Enzymes Require Inorganic Cations, p 193 • Enzymes requiring metal ions for full activity: (1) Metal-activated enzymes (2) Metalloenzymes Prentice Hall c 2002 Chapter 7 6

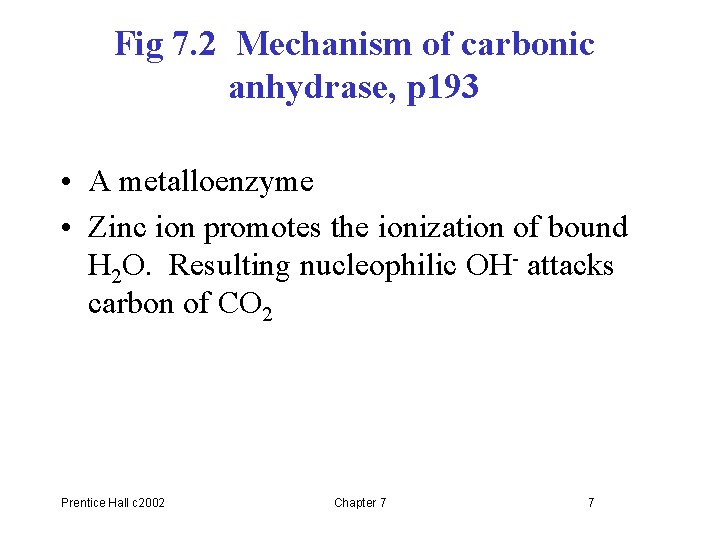
Fig 7. 2 Mechanism of carbonic anhydrase, p 193 • A metalloenzyme • Zinc ion promotes the ionization of bound H 2 O. Resulting nucleophilic OH- attacks carbon of CO 2 Prentice Hall c 2002 Chapter 7 7

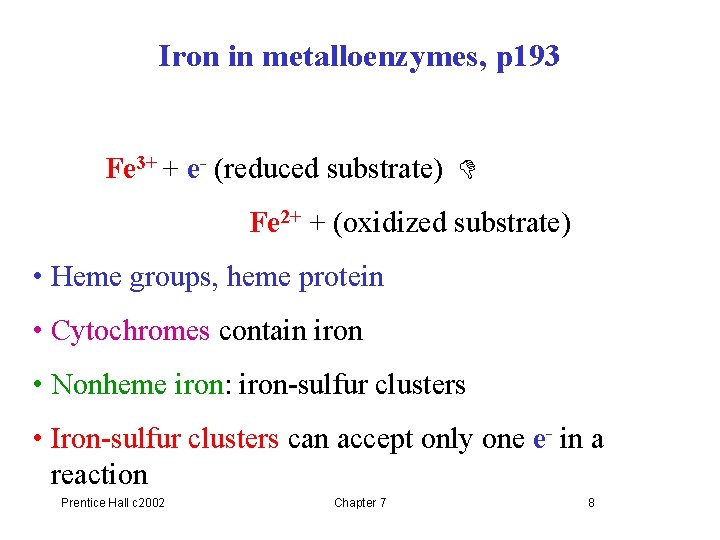
Iron in metalloenzymes, p 193 Fe 3+ + e- (reduced substrate) Fe 2+ + (oxidized substrate) • Heme groups, heme protein • Cytochromes contain iron • Nonheme iron: iron-sulfur clusters • Iron-sulfur clusters can accept only one e- in a reaction Prentice Hall c 2002 Chapter 7 8

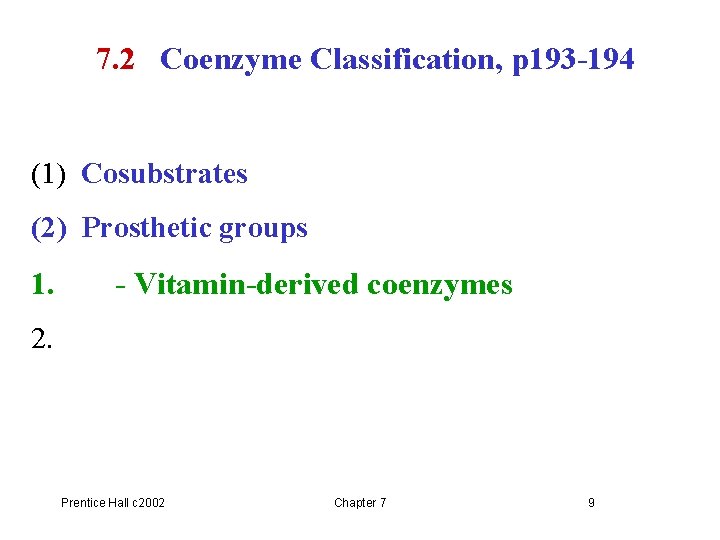
7. 2 Coenzyme Classification, p 193 -194 (1) Cosubstrates (2) Prosthetic groups 1. - Vitamin-derived coenzymes 2. Prentice Hall c 2002 Chapter 7 9

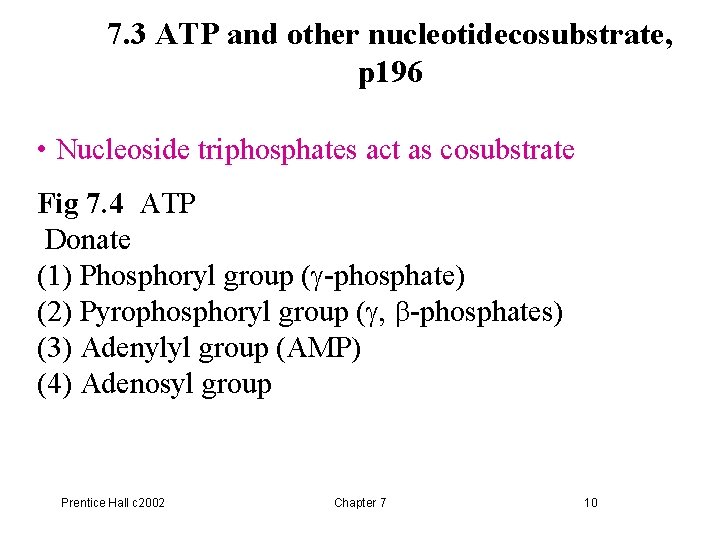
7. 3 ATP and other nucleotidecosubstrate, p 196 • Nucleoside triphosphates act as cosubstrate Fig 7. 4 ATP Donate (1) Phosphoryl group (g-phosphate) (2) Pyrophosphoryl group (g, b-phosphates) (3) Adenylyl group (AMP) (4) Adenosyl group Prentice Hall c 2002 Chapter 7 10

S-adenosylmethionine synthesis, p 196 • ATP is also a source of other metabolite coenzymes such as S-adenosylmethionine • Equation 7. 1 • S-adenosylmethionine donates methyl groups in many biosynthesis reactions – Synthesis of the hormone epinephrine from norepinephrine – Equation 7. 2 Prentice Hall c 2002 Chapter 7 11

Nucleotide-sugar coenzymes are involved in carbohydrate metabolism • UDP-Glucose is a sugar coenzyme • Fig 7. 6, p 197 Prentice Hall c 2002 Chapter 7 12

Vitamin-Derived Coenzymes and Nutrition, p 194 • Animals rely on plants and microorganisms for vitamin sources (meat supplies vitamins also) • Most vitamins must be enzymatically transformed to the coenzyme • Table 7. 1 Vitamins, nutritional deficiency diseases, p 194 Prentice Hall c 2002 Chapter 7 13

Box 7. 1 Vitamin C: a vitamin but not a coenzyme, p 195 • A reducing reagent for hydroxylation of collagen • Deficiency leads to the disease scurvy • Most animals (not primates) can synthesize Vit C • Anti-oxidant Prentice Hall c 2002 Chapter 7 14

7. 4 NAD+ and NADP+, p 197 • Vitamin: Nicotinic acid (niacin) • Coenzyme: NAD+ and NADP+ • Lack of niacin causes the disease pellagra • Humans obtain niacin from cereals, meat, legumes • Fig 7. 8 • Dehydrogenases transfer a hydride ion (H: -, one proton and two electrons) from a substrate to pyridine ring C-4 of NAD+ or NADP+ • The net reaction is: Prentice Hall c 2002+ 2 e- + 2 H+ Chapter 7 NAD(P)+ NAD(P)H + H+ 15

Reaction of lactate dehydrogenase Equation 7. 3 Fig 7. 9 Mechanism of lactate dehydrogenase, p 200 Prentice Hall c 2002 Chapter 7 16

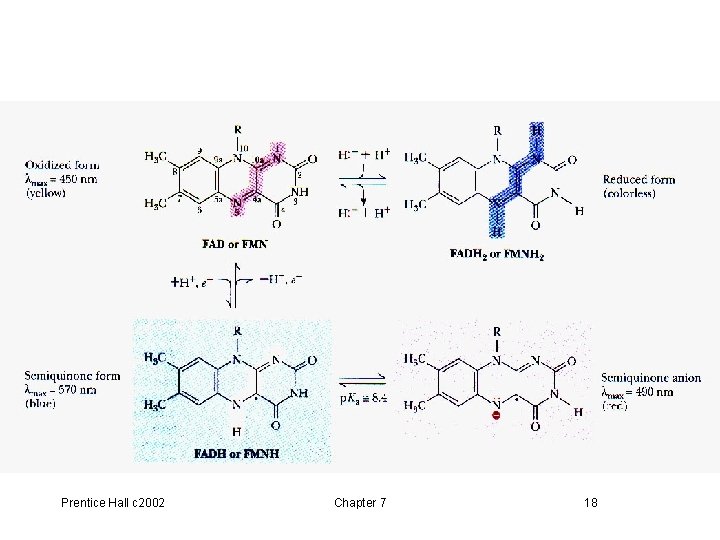
7. 5 FAD and FMN, p 200 -201 • Flavin adenine dinucleotide (FAD) • Flavin mono-nucleotide (FMN) • Derived from riboflavin (Vit B 2) • In oxidation-reduction reactions • One or two electron transfers • Fig 7. 10, Fig 7. 11 Prentice Hall c 2002 Chapter 7 17

Prentice Hall c 2002 Chapter 7 18

7. 6 Coenzyme A (Co. A or HS-Co. A) p 201 -202 • Derived from the vitamin pantothenate (Vit B 3) • Acyl-group transfer reactions • Acyl groups are covalently attached to the -SH of Co. A to form thioesters • Fig 7. 12, Fig. 7. 13 Prentice Hall c 2002 Chapter 7 19

7. 7 Thiamine Pyrophosphate (TPP) p 202 -203 • TPP is a derivative of thiamine (Vit B 1) • Reactive center: thiazolium ring • Fig 7. 14 • TPP participates in reactions of: (1) Decarboxylation (2) Oxidative decarboxylation of -keto acids (3) Transketolase enzyme reactions Prentice Hall c 2002 Chapter 7 20

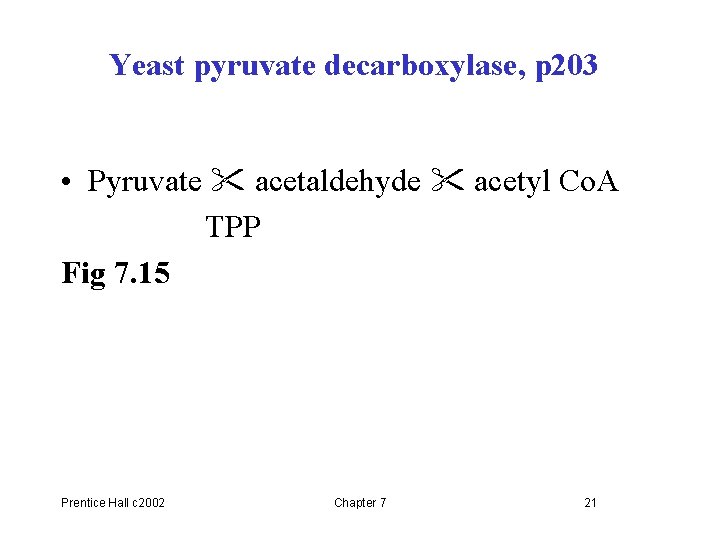
Yeast pyruvate decarboxylase, p 203 • Pyruvate acetaldehyde acetyl Co. A TPP Fig 7. 15 Prentice Hall c 2002 Chapter 7 21

7. 8 Pyridoxal Phosphate (PLP), p 203 -206 • Derived from Vit B 6 • Vitamin B 6 (Pyridoxine) is phosphorylated to form PLP • Involving amino acid metabolism (isomerizations, decarboxylations, side chain eliminations or replacements) • The reactive center is the aldehyde group • Fig 7. 16, Fig 7. 17 • Fig 7. 18 TPP in transaminase action Prentice Hall c 2002 Chapter 7 22

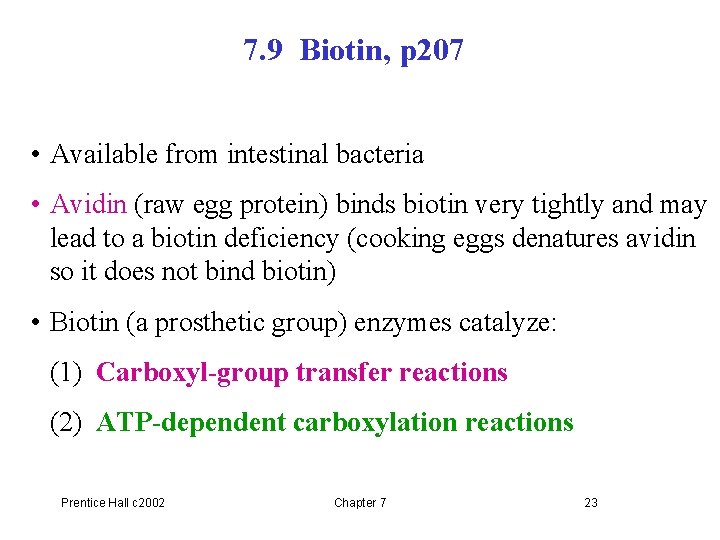
7. 9 Biotin, p 207 • Available from intestinal bacteria • Avidin (raw egg protein) binds biotin very tightly and may lead to a biotin deficiency (cooking eggs denatures avidin so it does not bind biotin) • Biotin (a prosthetic group) enzymes catalyze: (1) Carboxyl-group transfer reactions (2) ATP-dependent carboxylation reactions Prentice Hall c 2002 Chapter 7 23

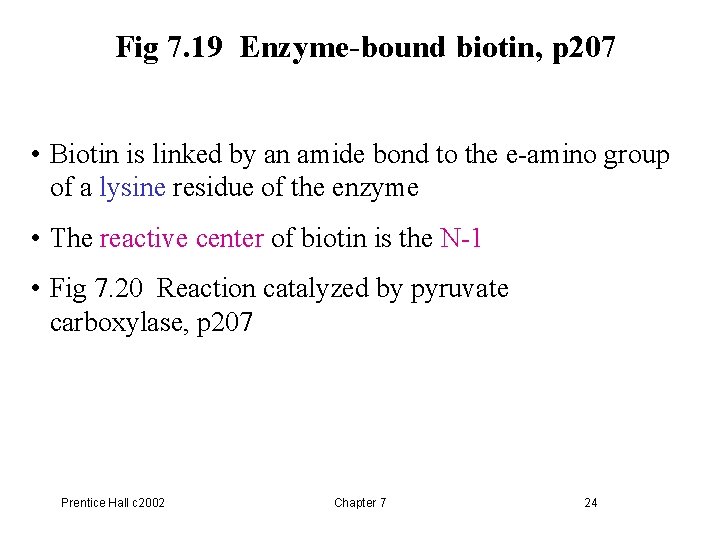
Fig 7. 19 Enzyme-bound biotin, p 207 • Biotin is linked by an amide bond to the e-amino group of a lysine residue of the enzyme • The reactive center of biotin is the N-1 • Fig 7. 20 Reaction catalyzed by pyruvate carboxylase, p 207 Prentice Hall c 2002 Chapter 7 24

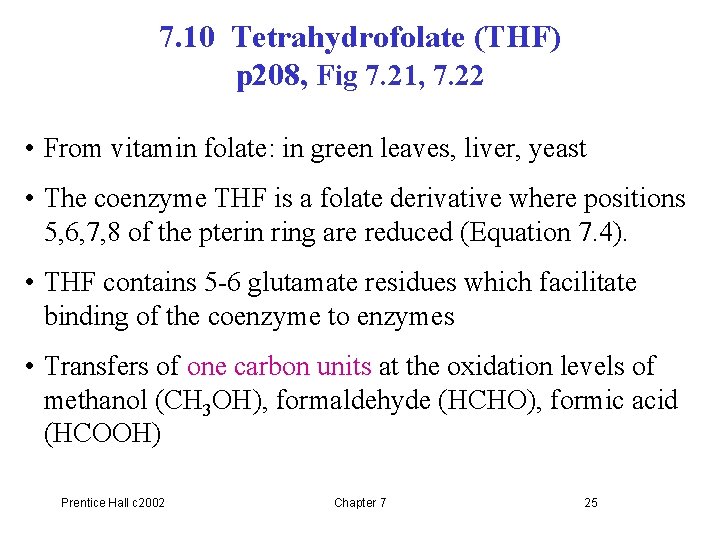
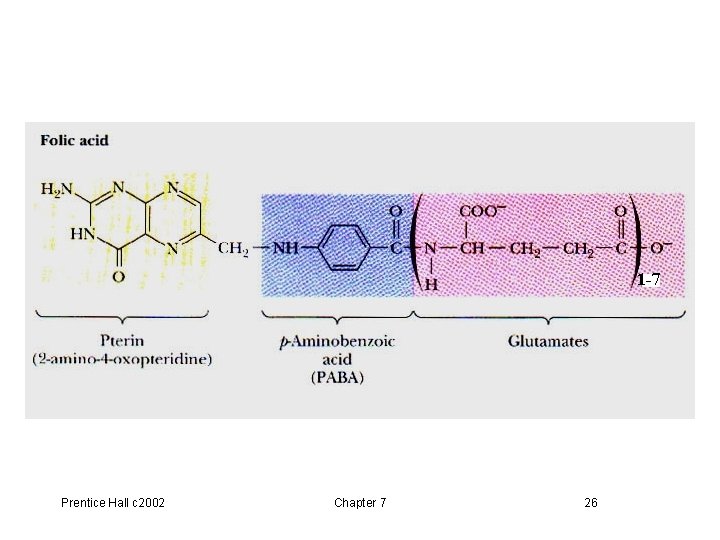
7. 10 Tetrahydrofolate (THF) p 208, Fig 7. 21, 7. 22 • From vitamin folate: in green leaves, liver, yeast • The coenzyme THF is a folate derivative where positions 5, 6, 7, 8 of the pterin ring are reduced (Equation 7. 4). • THF contains 5 -6 glutamate residues which facilitate binding of the coenzyme to enzymes • Transfers of one carbon units at the oxidation levels of methanol (CH 3 OH), formaldehyde (HCHO), formic acid (HCOOH) Prentice Hall c 2002 Chapter 7 25

1 -7 Prentice Hall c 2002 Chapter 7 26

Fig. 7. 23 5, 6, 7, 8, Tetrahydrobiopterin, a pterin coenzyme, p 210 • Coenzyme has a 3 -carbon side chain at C-6 • Not vitamin-derived, but synthesized by some organisms Prentice Hall c 2002 Chapter 7 27

7. 11 Cobalamin (Vitamin B 12), p 210 -211 • Coenzymes: methylcobalamin, adenosylcobalamin • Cobalamin contains a corrin ring system and a cobalt (it is synthesized by only a few microorganisms) • Humans obtain cobalamin from foods of animal origin (deficiency leads to pernicious anemia) • Coenzymes participate in enzyme-catalyzed molecular rearrangements • Fig. 7. 24 • Fig 7. 25 Intramolecular rearrangements catalyzed by Prentice Hall c 2002 Chapter 7 28 adenosylcobalamin enzymes, p 211

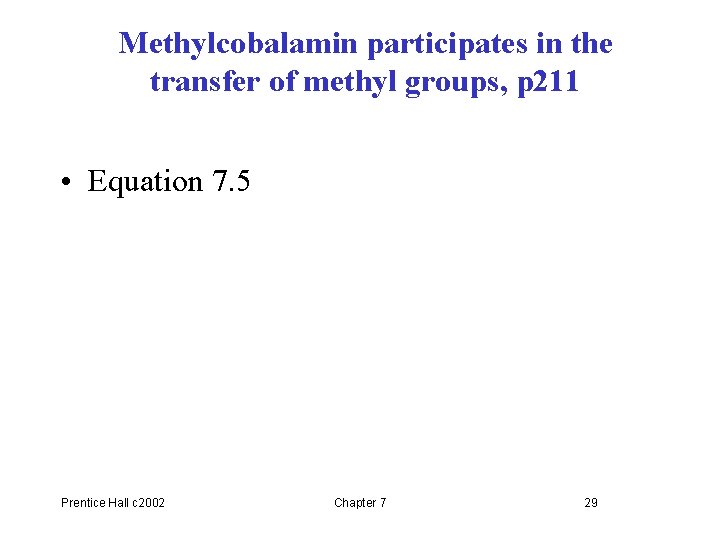
Methylcobalamin participates in the transfer of methyl groups, p 211 • Equation 7. 5 Prentice Hall c 2002 Chapter 7 29

7. 12 Lipoamide, p 212 • From lipoic acid • Coenzyme: lipoamide • Animals can synthesize lipoic acid, it is not a vitamin • Lipoic acid is an 8 -carbon carboxylic acid with sulfhydryl groups on C-6 and C-8 • Lipoamide functions as a “swinging arm” that carries acyl groups between active sites in multienzyme complexes Prentice Hall c 2002 Chapter 7 30

Fig 7. 26 Lipoamide, p 212 • Lipoic acid is bound via an amide linkage to the eamino group of an enzyme lysine • Transfer of an acyl group between active sites - Equation 7. 6 Prentice Hall c 2002 Chapter 7 31

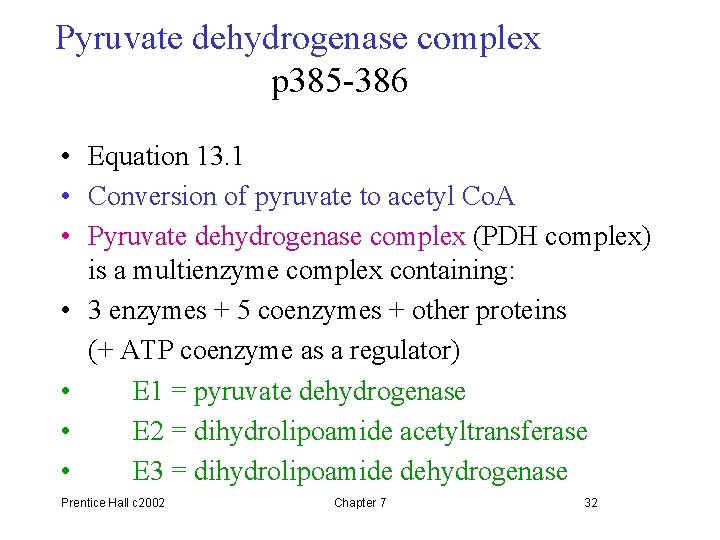
Pyruvate dehydrogenase complex p 385 -386 • Equation 13. 1 • Conversion of pyruvate to acetyl Co. A • Pyruvate dehydrogenase complex (PDH complex) is a multienzyme complex containing: • 3 enzymes + 5 coenzymes + other proteins (+ ATP coenzyme as a regulator) • E 1 = pyruvate dehydrogenase • E 2 = dihydrolipoamide acetyltransferase • E 3 = dihydrolipoamide dehydrogenase Prentice Hall c 2002 Chapter 7 32

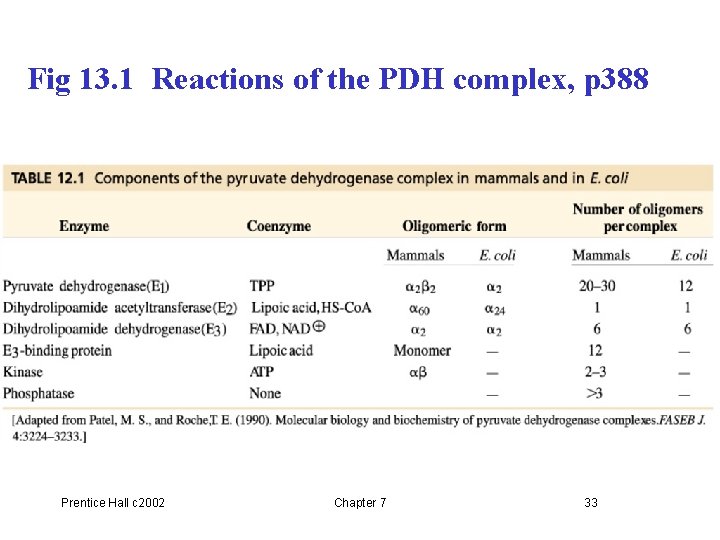
Fig 13. 1 Reactions of the PDH complex, p 388 Prentice Hall c 2002 Chapter 7 33

7. 13 Lipid Vitamins- p 212 -213 • Vitamin A, D, E, K • All contain rings and long, aliphatic side chains • Highly hydrophobic Prentice Hall c 2002 Chapter 7 34

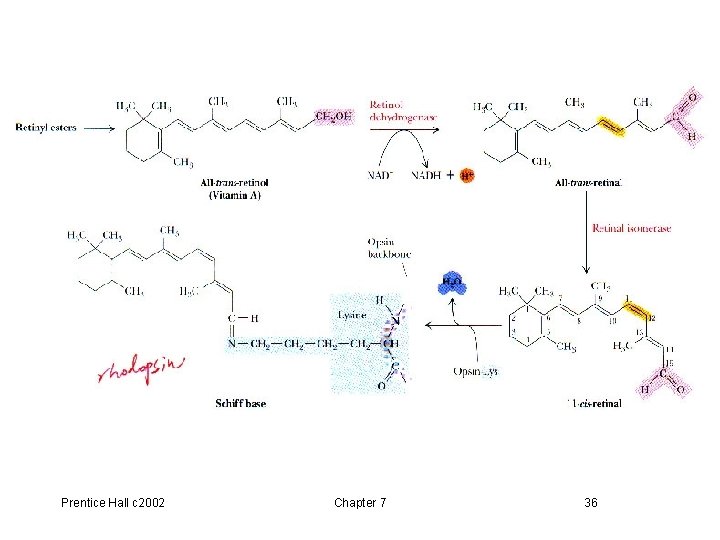
A. Vitamin A (Retinol), p 213 • Vit A exists in 3 forms: alcohol (retinol), aldehyde and retinoic acid • Retinol and retinoic acid are signal compounds • Rentinal (aldehyde) is a light-sensitive compound with a role in vision • Fig 7. 27 Prentice Hall c 2002 Chapter 7 35

Prentice Hall c 2002 Chapter 7 36

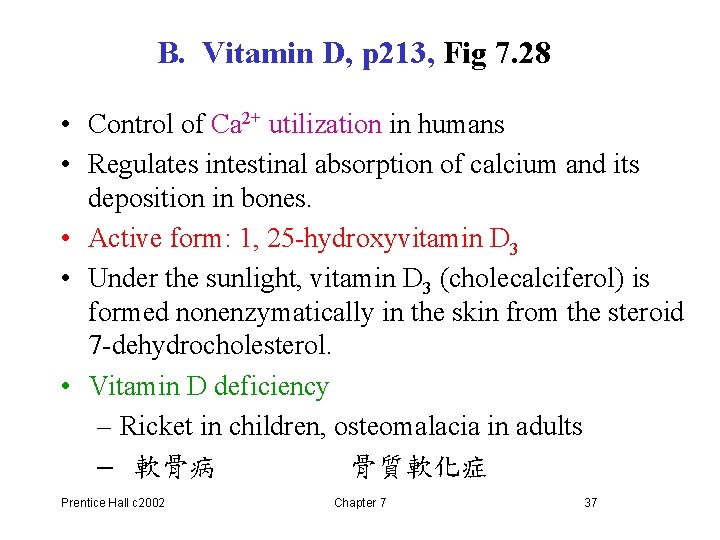
B. Vitamin D, p 213, Fig 7. 28 • Control of Ca 2+ utilization in humans • Regulates intestinal absorption of calcium and its deposition in bones. • Active form: 1, 25 -hydroxyvitamin D 3 • Under the sunlight, vitamin D 3 (cholecalciferol) is formed nonenzymatically in the skin from the steroid 7 -dehydrocholesterol. • Vitamin D deficiency – Ricket in children, osteomalacia in adults – 軟骨病 骨質軟化症 Prentice Hall c 2002 Chapter 7 37

Vitamin D, p 213 • Absorbed in the intestine or photosynthesized in the skin, cholecalciferol is transported to the liver by vitamin D-binding protein (DBP, or transcalciferin). • In the liver, cholecalciferol is 25 hydroxylated by mixed-function oxidase to form 25 -hydroxyvitamin D 3 Prentice Hall c 2002 Chapter 7 38

Vitamin D, p 213 • 25 -hydroxyvitamin D is the mayor circulating form of vitamin D in the body, but the biological activity is far less than the final active form, 1, 25 -hydroxyvitamin D 3 • In the kidney, a mitochondrial mixedfunction oxidase hydroxylates 25 hydroxyvitamin D to 1, 25 -hydroxyvitamin D 3 (Active form) Prentice Hall c 2002 Chapter 7 39

C. Vitamin E (a-tocopherol), p 213 • A reducing reagent that scavenges oxygen and free radicals • May prevent damage to fatty acids in membranes Fig 7. 29 Prentice Hall c 2002 Chapter 7 40

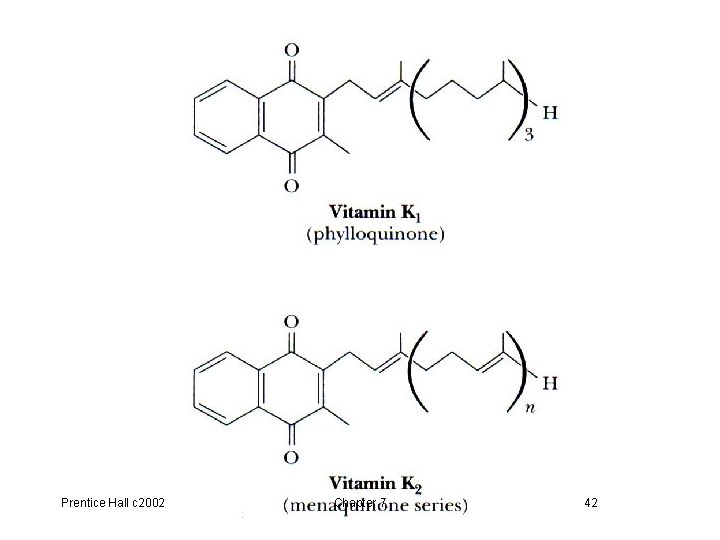
D. Vitamin K (phylloquinone), p 214 Fig 7. 29 • Required for synthesis of blood coagulation proteins • A coenzyme for mammalian carboxylases that convert glutamate to g-carboxyglutamate • Equation 7. 7 Vit K-dependent carboxylation, p 214 • Calcium binds to the g-carboxy. Glu residues of these coagulation proteins which adhere to platelet surfaces • Vitamin K analogs (used as competitive inhibitors to prevent regeneration of dihydrovitamin K) are given to individuals who suffer excessive blood clotting Prentice Hall c 2002 Chapter 7 41

Prentice Hall c 2002 Chapter 7 42

7. 14 Ubiquinone (Coenzyme Q), p 214 • Electrons transfer • Plastoquinone (ubiquinone analog) functions in photosynthetic electron transport • Hydrophobic tail: repeat of five-carbon isoprenoid units • Fig 7. 30, p 215 • Fig 7. 31, p 215 Prentice Hall c 2002 Chapter 7 43

7. 15 Protein Coenzymes , p 215 • Protein coenzymes (group-transfer proteins) • Participate in: (1) Group-transfer reactions (2) Oxidation-reduction reactions: transfer a hydrogen or an electron • Metal ions, iron-sulfur clusters and heme groups are commonly found in these proteins • Fig 7. 32 Thioredoxin, p 216 Prentice Hall c 2002 Chapter 7 44

7. 16 Cytochromes, p 216 • Heme-containing coenzymes • Fe(III) undergoes reversible one-electron reduction • Cytochromes a, b and c have different visible absorption spectra and heme prosthetic groups • Electron transfer potential varies among different cytochromes due to the different protein environment of each prosthetic group • Fig 7. 33 Heme group of cyt a, b, and c p 217 • Fig 7. 34 Absorption spectra of oxidized and reduced Prentice Hall c 2002 cytochrome c, Chapter 7 45 p 218