Chapter 3 Chemical Periodicity and The Formation of





- Slides: 17

Chapter 3 Chemical Periodicity and The Formation of Simple Compounds 3. 1 3. 2 3. 3 3. 4 3. 5 3. 6 3. 7 3. 8 Groups of Elements The Periodic Table Ions and Ionic Compounds Covalent Bonding and Lewis Structures Drawing Lewis Structures Naming Compounds in Which Covalent Bonding Occurs The Shapes of Molecules Elements Forming More than One Ion Topics to be emphasized in Exam 1

Chapter 3 Chemical Periodicity. Formation of Simple Compounds. Molecular Structure. Section 3. 2 The Periodic Table Section 3. 3 Covalent Bonding Section 3. 3 Lewis Structures Section 3. 4 Shapes of Molecules

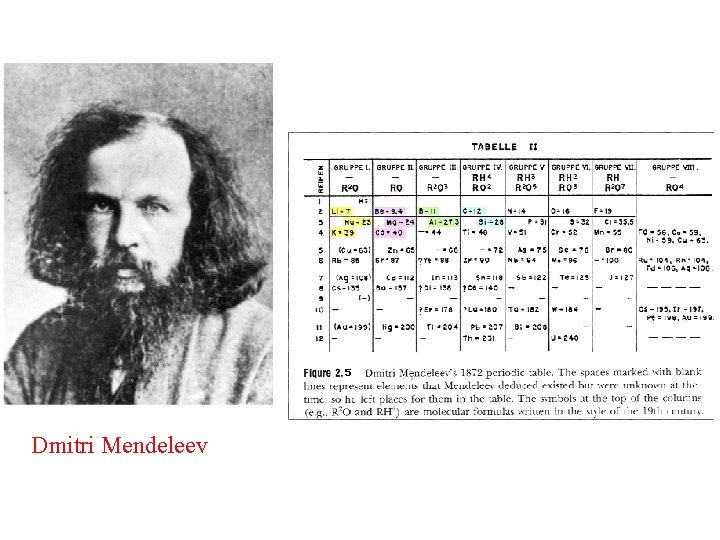
Section 3. 2 Periodic Table (1) Classic exemplar of the scientific process: Mendeleev (2) Atomic mass and atomic number as atom identifiers (3) Periodic properties along rows and down columns (4) Electronegativity (ability of an atom to hold electrons) (5) Chemical reactivity (kinds of reactions atoms undergo) (6) Valence (the number of bonds to other atoms) (7) (4) Underlying structure of the Periodic Table is the

Dmitri Mendeleev

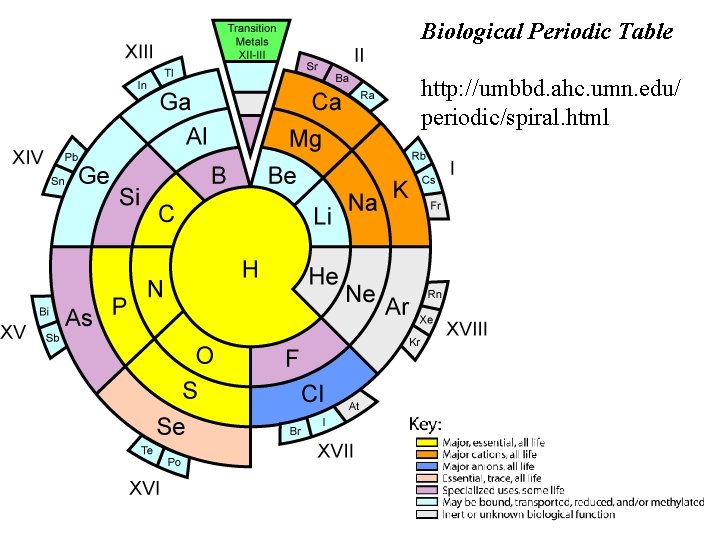
Biological Periodic Table http: //umbbd. ahc. umn. edu/ periodic/spiral. html


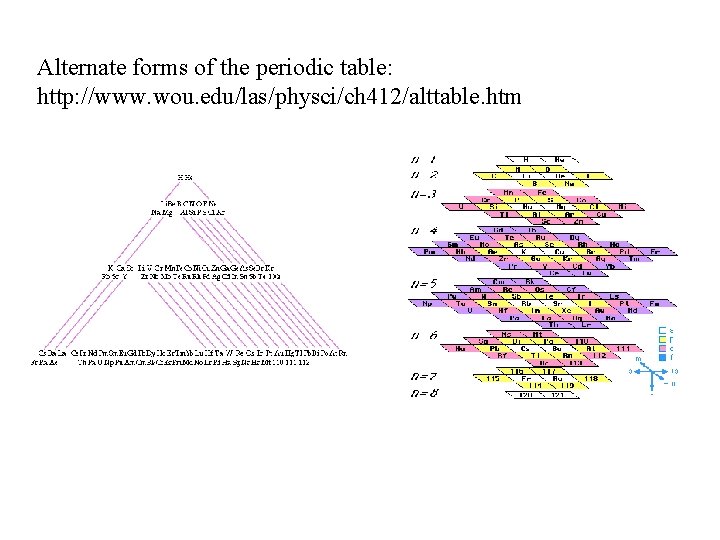
Alternate forms of the periodic table: http: //www. wou. edu/las/physci/ch 412/alttable. htm

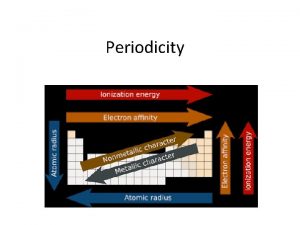
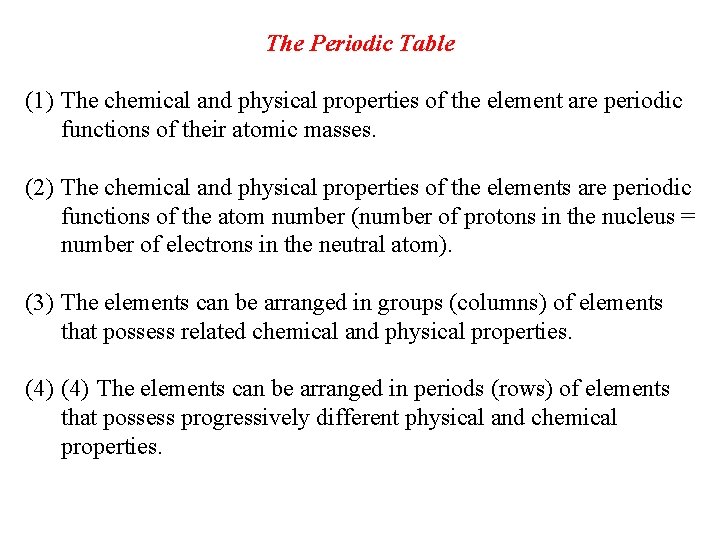
The Periodic Table (1) The chemical and physical properties of the element are periodic functions of their atomic masses. (2) The chemical and physical properties of the elements are periodic functions of the atom number (number of protons in the nucleus = number of electrons in the neutral atom). (3) The elements can be arranged in groups (columns) of elements that possess related chemical and physical properties. (4) The elements can be arranged in periods (rows) of elements that possess progressively different physical and chemical properties.


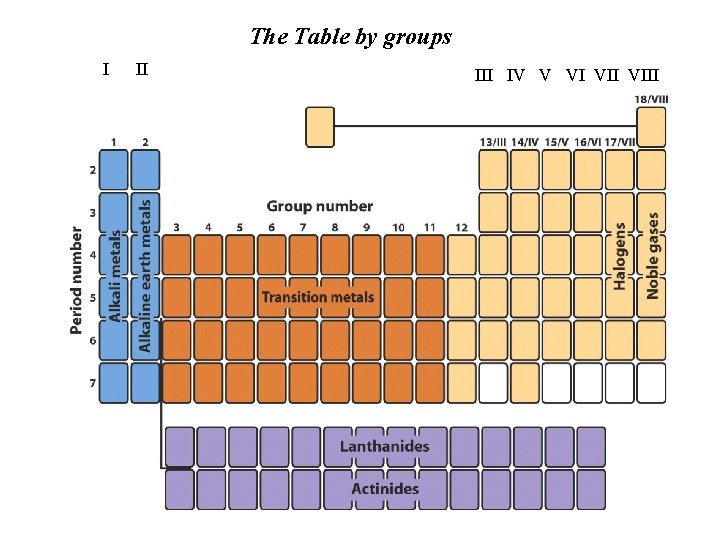
The Table by groups I II IV V VI VIII

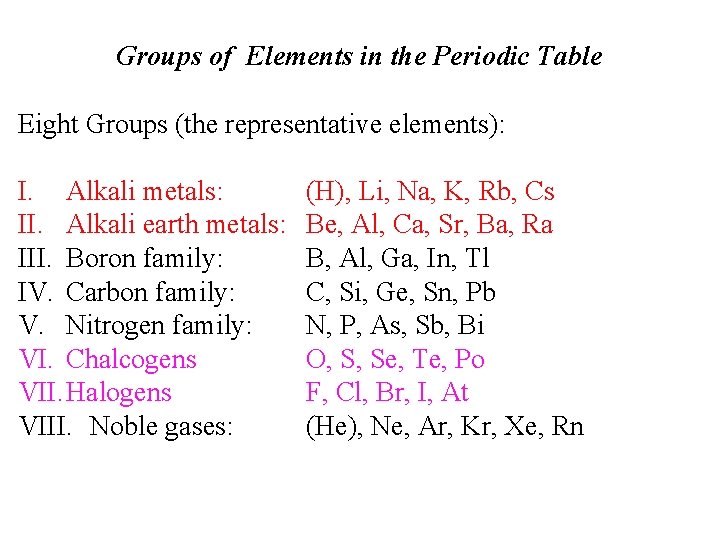
Groups of Elements in the Periodic Table Eight Groups (the representative elements): I. Alkali metals: II. Alkali earth metals: III. Boron family: IV. Carbon family: V. Nitrogen family: VI. Chalcogens VII. Halogens VIII. Noble gases: (H), Li, Na, K, Rb, Cs Be, Al, Ca, Sr, Ba, Ra B, Al, Ga, In, Tl C, Si, Ge, Sn, Pb N, P, As, Sb, Bi O, S, Se, Te, Po F, Cl, Br, I, At (He), Ne, Ar, Kr, Xe, Rn

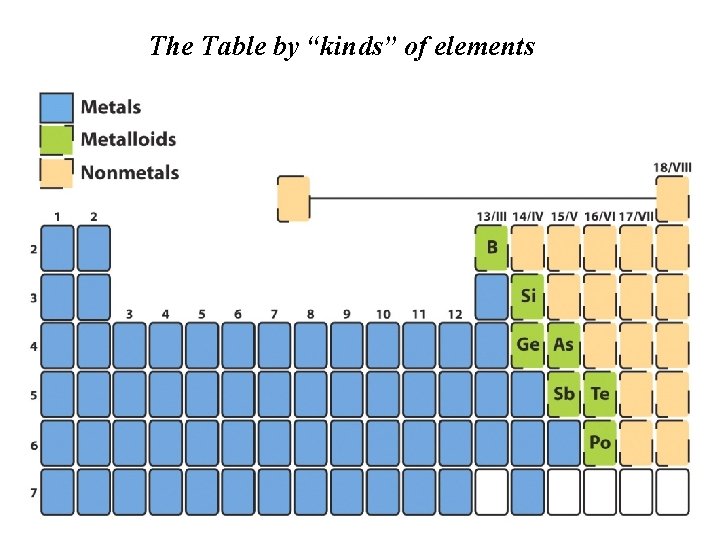
The Table by “kinds” of elements

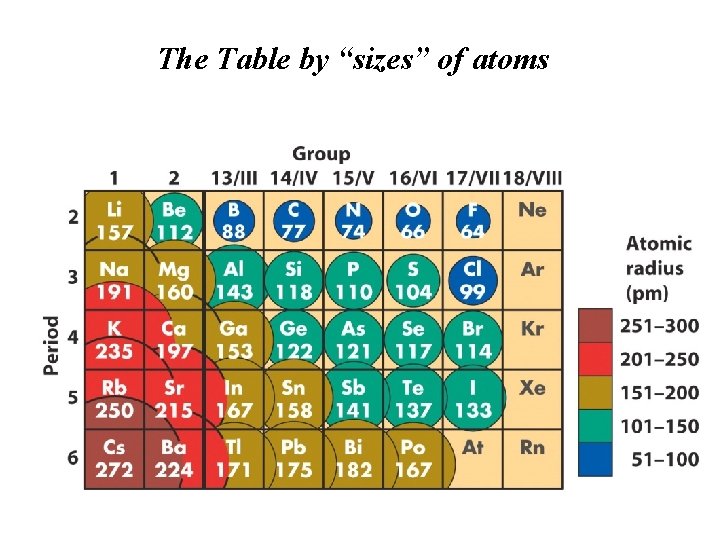
The Table by “sizes” of atoms

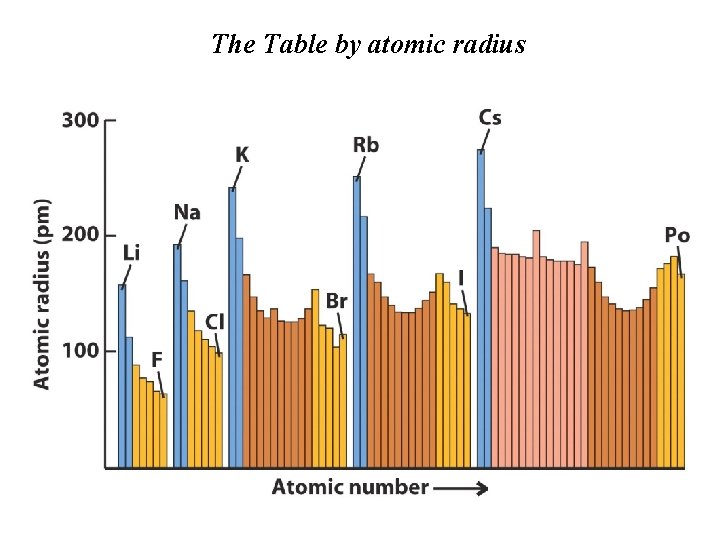
The Table by atomic radius

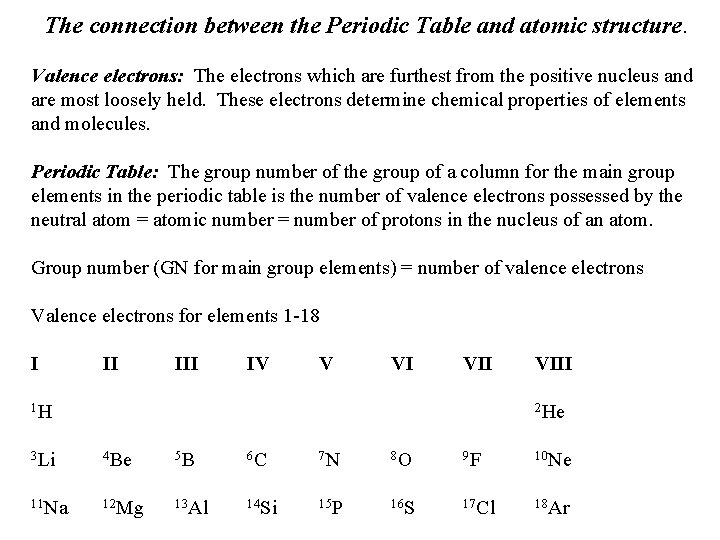
The connection between the Periodic Table and atomic structure. Valence electrons: The electrons which are furthest from the positive nucleus and are most loosely held. These electrons determine chemical properties of elements and molecules. Periodic Table: The group number of the group of a column for the main group elements in the periodic table is the number of valence electrons possessed by the neutral atom = atomic number = number of protons in the nucleus of an atom. Group number (GN for main group elements) = number of valence electrons Valence electrons for elements 1 -18 I II IV V VI VII 1 H VIII 2 He 3 Li 4 Be 5 B 6 C 7 N 8 O 9 F 10 Ne 11 Na 12 Mg 13 Al 14 Si 15 P 16 S 17 Cl 18 Ar

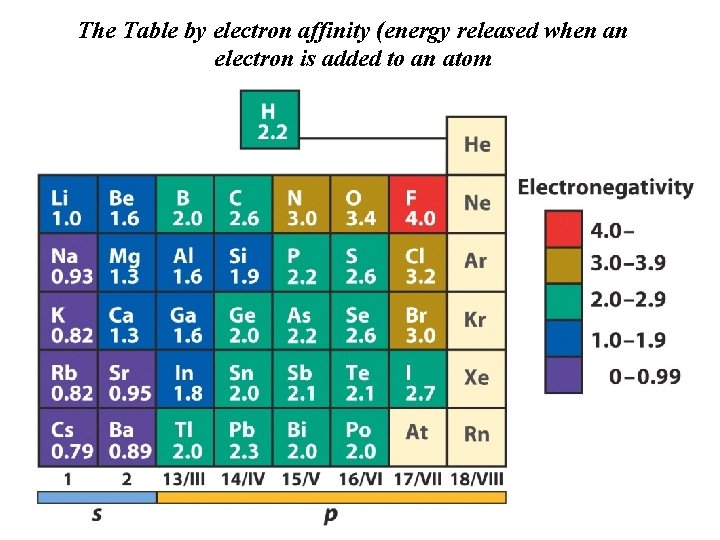
The Table by electron affinity (energy released when an electron is added to an atom

Electronegativity and electron affinity are two key features which determine the nature of the chemical bond. More later….
 Electronic configuration of mo (z=42)
Electronic configuration of mo (z=42) Oxygen periodic trends
Oxygen periodic trends Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity 8 electron configuration
8 electron configuration Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity What is periodicity
What is periodicity First dental home visit documentation form
First dental home visit documentation form Monophagic
Monophagic Chemsheets periodicity
Chemsheets periodicity Aap bright futures periodicity schedule
Aap bright futures periodicity schedule Electronegativity
Electronegativity Filariasis
Filariasis Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Are kc and kp equal
Are kc and kp equal Formation initiale vs formation continue
Formation initiale vs formation continue Chemical change formation of precipitate
Chemical change formation of precipitate Chemical change formation of precipitate
Chemical change formation of precipitate