Chapter 27 Quantum Physics Quantum Physics II Sections













- Slides: 26

Chapter 27 Quantum Physics

Quantum Physics II Sections 4– 8 General Physics

Diffraction of X-rays by Crystals w Diffraction of x-rays can occur if the spacing between the lines is approximately equal to the wavelength of the x-ray radiation w The regular array of atoms in a crystal can act as a three-dimensional grating for diffracting x -rays General Physics

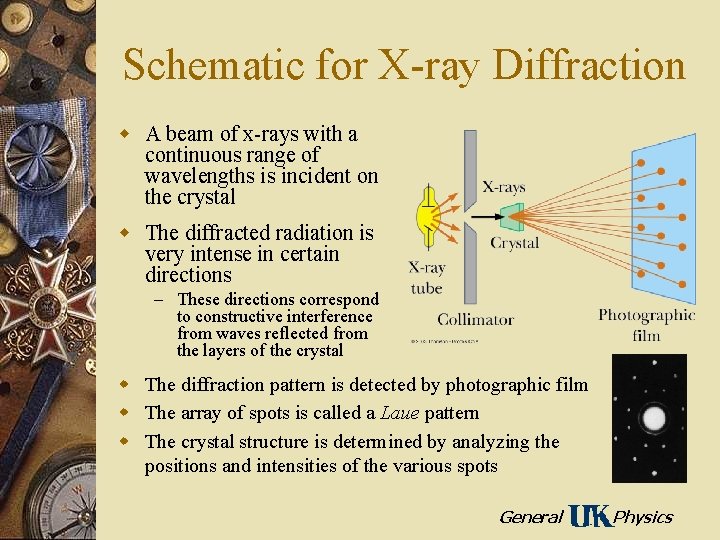
Schematic for X-ray Diffraction w A beam of x-rays with a continuous range of wavelengths is incident on the crystal w The diffracted radiation is very intense in certain directions – These directions correspond to constructive interference from waves reflected from the layers of the crystal w The diffraction pattern is detected by photographic film w The array of spots is called a Laue pattern w The crystal structure is determined by analyzing the positions and intensities of the various spots General Physics

X-ray Diffraction & DNA Structure w The main technique used to determine the molecular structure of proteins, DNA, and RNA is x-ray diffraction using x-rays of wavelength of about 0. 1 nm General Physics

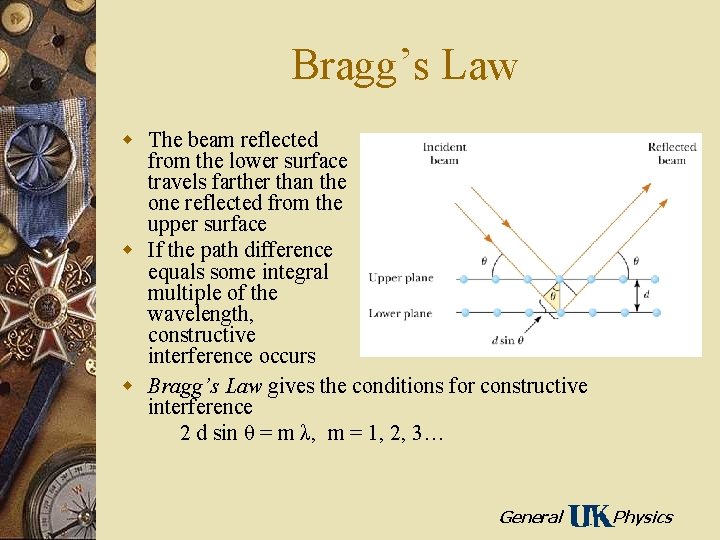
Bragg’s Law w The beam reflected from the lower surface travels farther than the one reflected from the upper surface w If the path difference equals some integral multiple of the wavelength, constructive interference occurs w Bragg’s Law gives the conditions for constructive interference 2 d sin θ = m λ, m = 1, 2, 3… General Physics

Arthur Holly Compton w 1892 – 1962 w Discovered the Compton effect w Worked with cosmic rays w Director of Laboratory at University of Chicago w Shared Nobel Prize in 1927 General Physics

The Compton Effect w Compton directed a beam of x-rays toward a block of graphite w He found that the scattered x-rays had a slightly longer wavelength that the incident x-rays – This means they also had less energy w The amount of energy reduction depended on the angle at which the x-rays were scattered w The change in wavelength is called the Compton shift General Physics

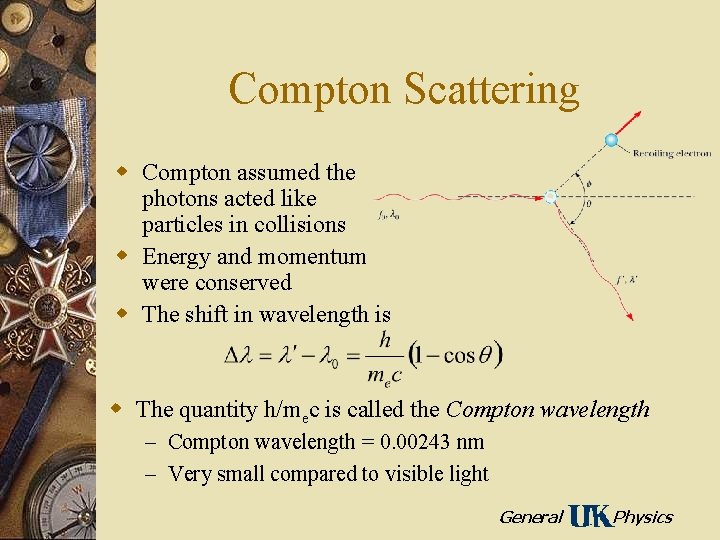
Compton Scattering w Compton assumed the photons acted like particles in collisions w Energy and momentum were conserved w The shift in wavelength is w The quantity h/mec is called the Compton wavelength – Compton wavelength = 0. 00243 nm – Very small compared to visible light General Physics

Dual Nature of Light w Light has a dual nature. It exhibits both wave and particle characteristics – Applies to all electromagnetic radiation – Different frequencies allow one or the other characteristic to be more easily observed w The photoelectric effect and Compton scattering offer evidence for the particle nature of light – When light and matter interact, light behaves as if it were composed of particles w Interference and diffraction offer evidence of the wave nature of light General Physics

Louis de Broglie w 1892 – 1987 w Discovered the wave nature of electrons w Awarded Nobel Prize in 1929 General Physics

de Broglie’s Hypothesis w In 1924, Louis de Broglie postulated that because photons have wave and particle properties, perhaps matter also has both a particle and wave nature w Furthermore, the wavelength and frequency of matter waves can be determined w Recall photons have an energy given by the relations E = pc (Einstein’s special relativity) E = hf = hc/λ (Einstein’s photoelectric effect) w So the wavelength of a photon can be expressed as General Physics

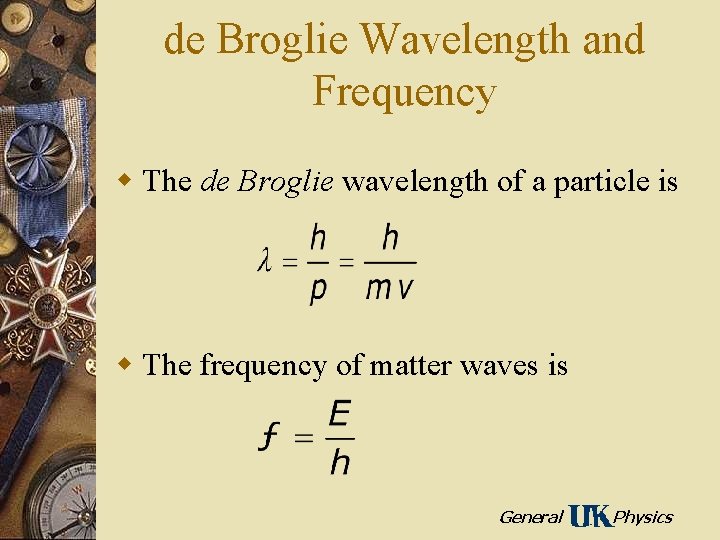
de Broglie Wavelength and Frequency w The de Broglie wavelength of a particle is w The frequency of matter waves is General Physics

Dual Nature of Matter w The de Broglie equations show the dual nature of matter w Each contains matter concepts – Energy and momentum w Each contains wave concepts – Wavelength and frequency w The Davisson-Germer Experiment confirmed the wave nature of electrons – Scattered low-energy electrons from a nickel target and observed a diffraction pattern – The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength General Physics

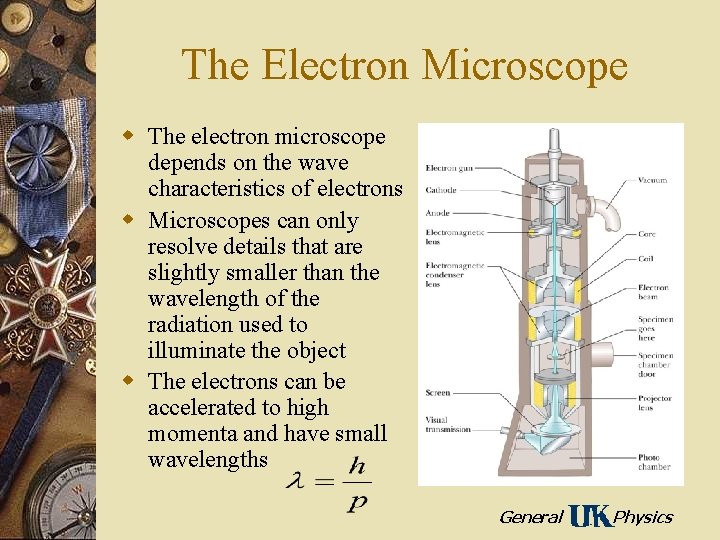
The Electron Microscope w The electron microscope depends on the wave characteristics of electrons w Microscopes can only resolve details that are slightly smaller than the wavelength of the radiation used to illuminate the object w The electrons can be accelerated to high momenta and have small wavelengths General Physics

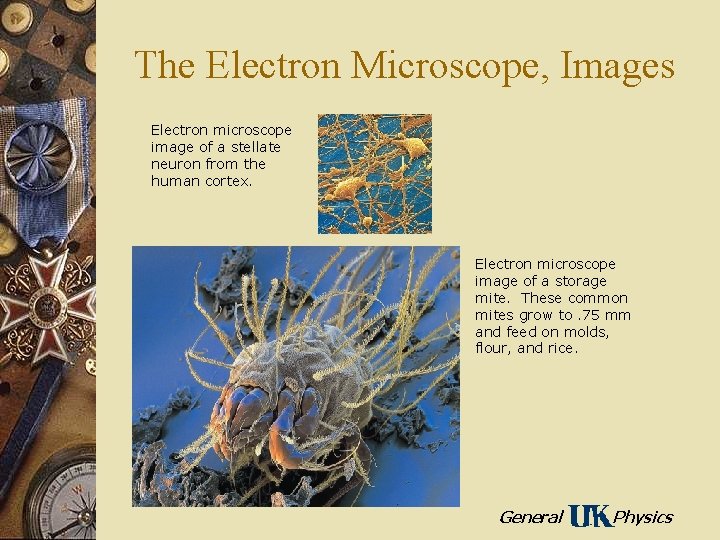
The Electron Microscope, Images Electron microscope image of a stellate neuron from the human cortex. Electron microscope image of a storage mite. These common mites grow to. 75 mm and feed on molds, flour, and rice. General Physics

Microscope Resolutions w In ordinary microscopes, the resolution is limited by the wavelength of the waves used to make the image – Optical, resolution is about 200 nm – Electron, resolution is about 0. 2 nm • Need high energy • Would penetrate the target, so not good for surface details General Physics

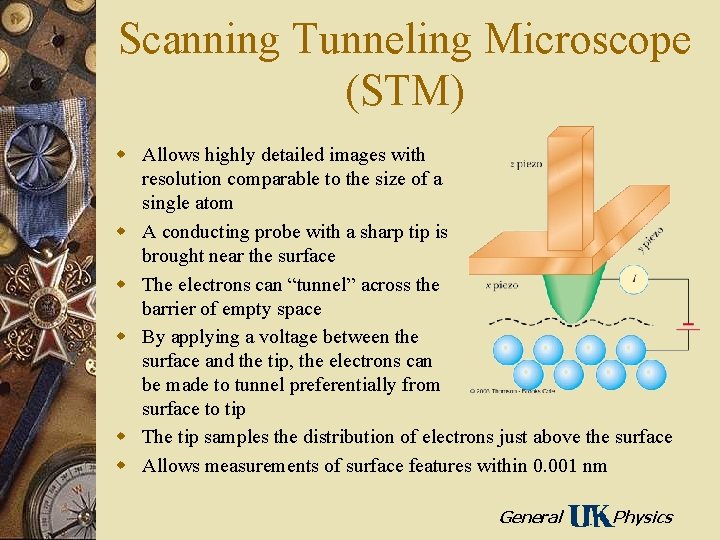
Scanning Tunneling Microscope (STM) w Allows highly detailed images with resolution comparable to the size of a single atom w A conducting probe with a sharp tip is brought near the surface w The electrons can “tunnel” across the barrier of empty space w By applying a voltage between the surface and the tip, the electrons can be made to tunnel preferentially from surface to tip w The tip samples the distribution of electrons just above the surface w Allows measurements of surface features within 0. 001 nm General Physics

STM Result, Example w This is a “quantum corral” of 48 iron atoms on a copper surface w The diameter of the ring is 143 nm w Obtained with a low temperature STM General Physics

Erwin Schrödinger w 1887 – 1961 w Best known as the creator of wave mechanics w Worked on problems in general relativity, cosmology, and the application of quantum mechanics to biology General Physics

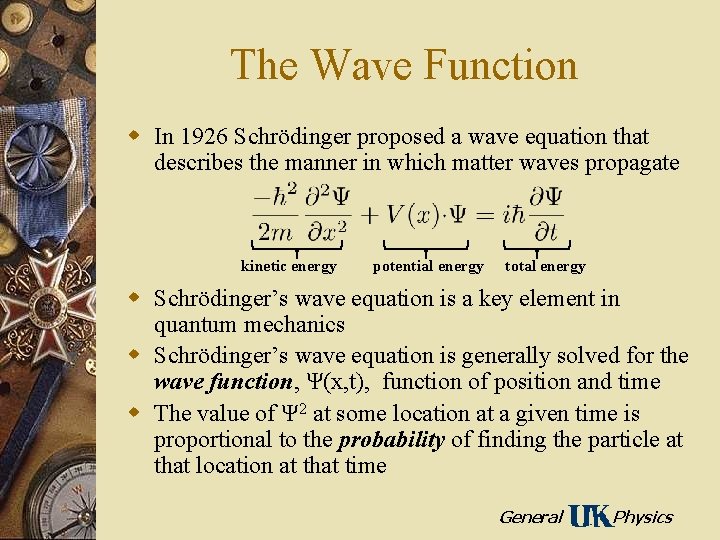
The Wave Function w In 1926 Schrödinger proposed a wave equation that describes the manner in which matter waves propagate kinetic energy potential energy total energy w Schrödinger’s wave equation is a key element in quantum mechanics w Schrödinger’s wave equation is generally solved for the wave function, Ψ(x, t), function of position and time w The value of Ψ 2 at some location at a given time is proportional to the probability of finding the particle at that location at that time General Physics

Werner Heisenberg w 1901 – 1976 w Developed an abstract mathematical model to explain wavelengths of spectral lines – Called matrix mechanics w Other contributions – Uncertainty Principle • Nobel Prize in 1932 – Atomic and nuclear models – Forms of molecular hydrogen General Physics

The Uncertainty Principle w When measurements are made, the experimenter is always faced with experimental uncertainties in the measurements – Classical mechanics offers no fundamental barrier to ultimate refinements in measurements – Classical mechanics would allow for measurements with infinitesimally small uncertainties w Quantum mechanics predicts that a barrier to measurements with ultimately small uncertainties does exist w In 1927 Heisenberg introduced the uncertainty principle – If a measurement of position of a particle is made with precision Δx and a simultaneous measurement of linear momentum is made with precision Δpx, then the product of the two uncertainties can never be smaller than h/4 General Physics

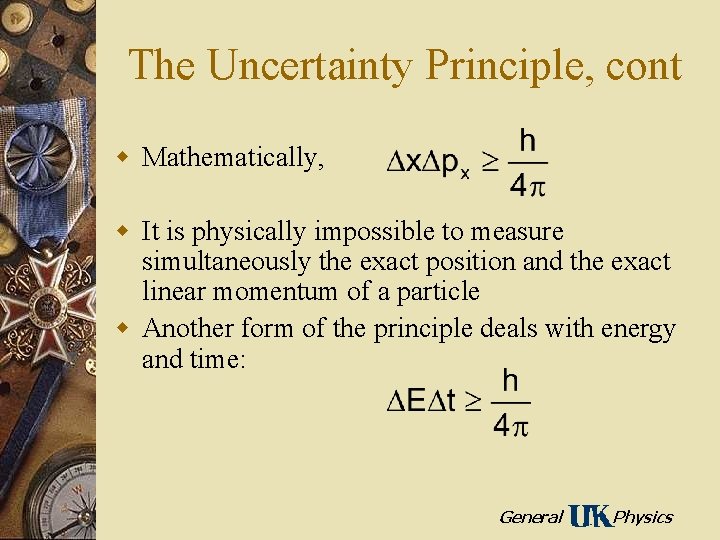
The Uncertainty Principle, cont w Mathematically, w It is physically impossible to measure simultaneously the exact position and the exact linear momentum of a particle w Another form of the principle deals with energy and time: General Physics

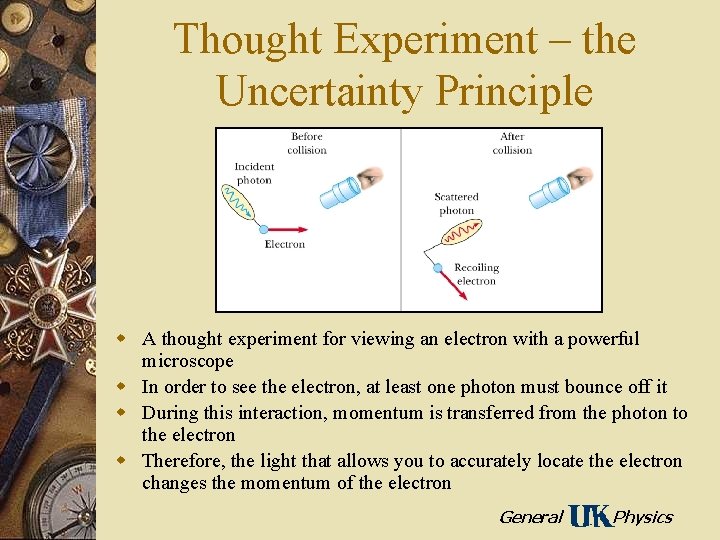
Thought Experiment – the Uncertainty Principle w A thought experiment for viewing an electron with a powerful microscope w In order to see the electron, at least one photon must bounce off it w During this interaction, momentum is transferred from the photon to the electron w Therefore, the light that allows you to accurately locate the electron changes the momentum of the electron General Physics

Uncertainty Principle Applied to an Electron w View the electron as a particle w Its position and velocity cannot both be know precisely at the same time w Its velocity can be uncertain over a range in position given by Δx ≈ h / (4π mΔv) w Its time and energy cannot both be know precisely at the same time w Its energy can be uncertain for a period given by Δt ≈ h / (4π ΔE) General Physics