Chapter 27 Quantum Physics Quantum Physics I Sections








- Slides: 29

Chapter 27 Quantum Physics

Quantum Physics I Sections 1– 3 General Physics

Need for Quantum Physics w Other problems remained in classical physics which relativity did not explain w Blackbody Radiation – The electromagnetic radiation emitted by a heated object w Photoelectric Effect – Emission of electrons by an illuminated metal w Compton Effect – A beam of x-rays directed toward a block of graphite scattered at a slightly longer wavelength w Spectral Lines – Emission of sharp spectral lines by gas atoms in an electric discharge tube General Physics

Development of Quantum Physics w Beginning in 1900 – Development of ideas of quantum physics • Also called wave mechanics • Highly successful in explaining the behavior of atoms, molecules, and nuclei w Involved a large number of physicists General Physics

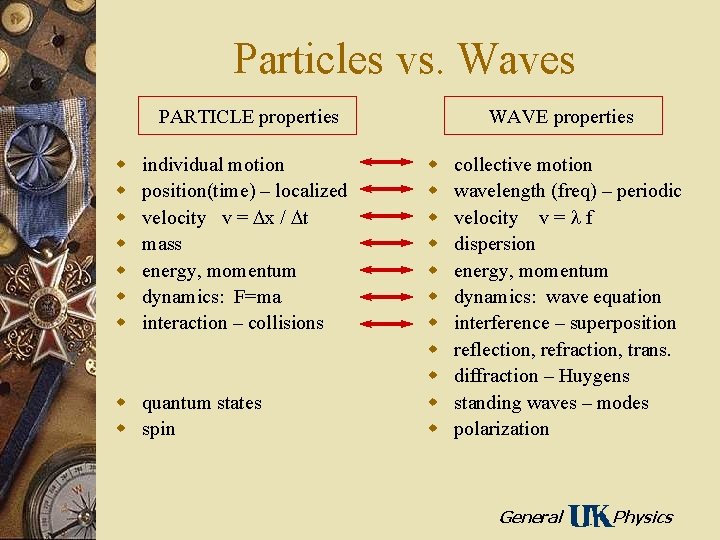
Particles vs. Waves PARTICLE properties w w w w individual motion position(time) – localized velocity v = x / t mass energy, momentum dynamics: F=ma interaction – collisions w quantum states w spin WAVE properties w w w collective motion wavelength (freq) – periodic velocity v = f dispersion energy, momentum dynamics: wave equation interference – superposition reflection, refraction, trans. diffraction – Huygens standing waves – modes polarization General Physics

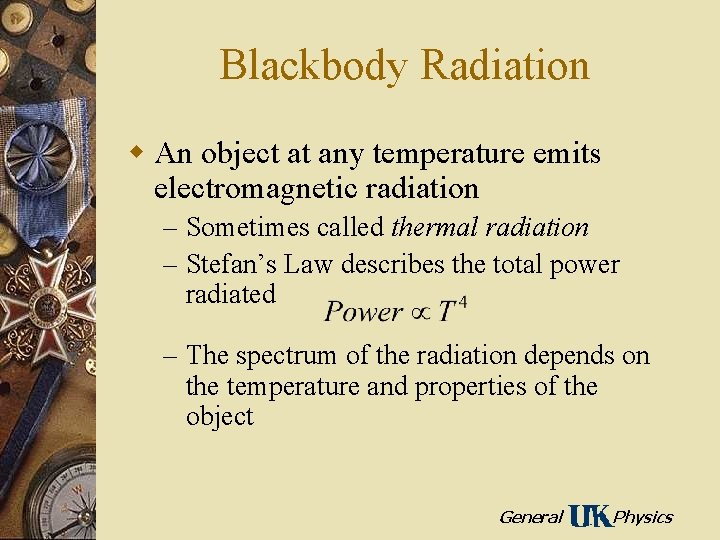
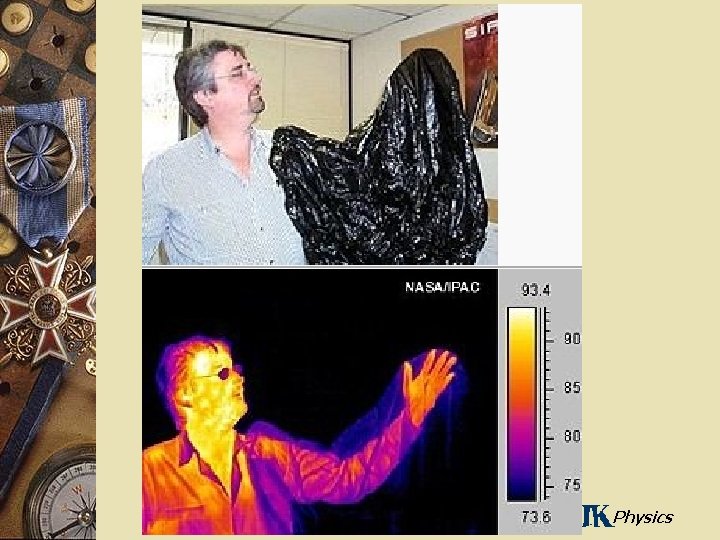
Blackbody Radiation w An object at any temperature emits electromagnetic radiation – Sometimes called thermal radiation – Stefan’s Law describes the total power radiated – The spectrum of the radiation depends on the temperature and properties of the object General Physics

General Physics

General Physics

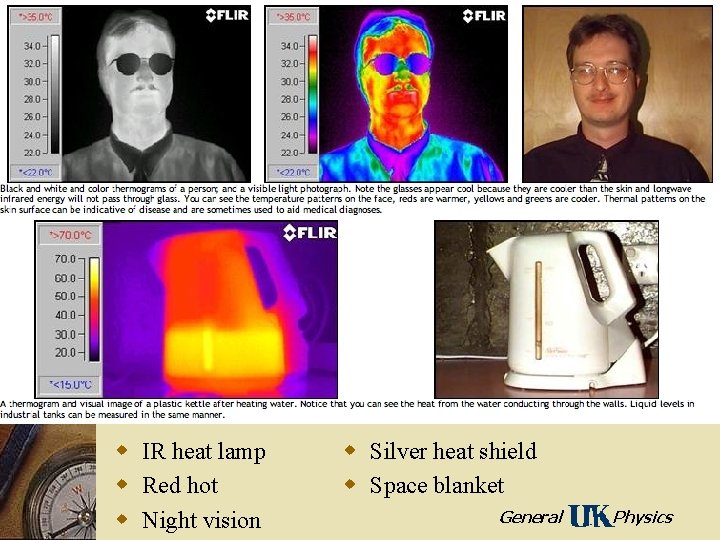
w IR heat lamp w Red hot w Night vision w Silver heat shield w Space blanket General Physics

General Physics

General Physics

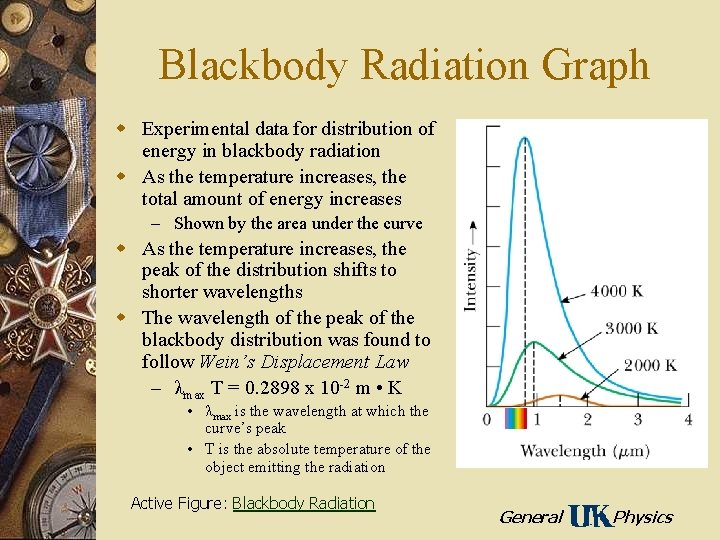
Blackbody Radiation Graph w Experimental data for distribution of energy in blackbody radiation w As the temperature increases, the total amount of energy increases – Shown by the area under the curve w As the temperature increases, the peak of the distribution shifts to shorter wavelengths w The wavelength of the peak of the blackbody distribution was found to follow Wein’s Displacement Law – λmax T = 0. 2898 x 10 -2 m • K • λmax is the wavelength at which the curve’s peak • T is the absolute temperature of the object emitting the radiation Active Figure: Blackbody Radiation General Physics

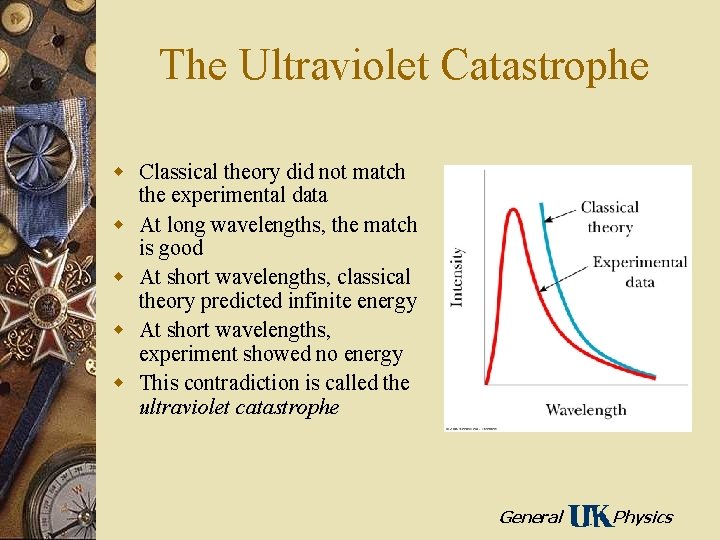
The Ultraviolet Catastrophe w Classical theory did not match the experimental data w At long wavelengths, the match is good w At short wavelengths, classical theory predicted infinite energy w At short wavelengths, experiment showed no energy w This contradiction is called the ultraviolet catastrophe General Physics

Max Planck w 1858 – 1947 w Introduced a “quantum of action” now known as Planck’s constant w Awarded Nobel Prize in 1918 for discovering the quantized nature of energy General Physics

Planck’s Resolution w Planck hypothesized that the blackbody radiation was produced by resonators – Resonators were submicroscopic charged oscillators w The resonators could only have discrete energies – En = n h ƒ • n is called the quantum number • ƒ is the frequency of vibration • h is Planck’s constant, h = 6. 626 x 10 -34 J s w Key Point: quantized energy states w Few high energy resonators populated Active Figure: Planck's Quantized Energy States General Physics

Photoelectric Effect w When light is incident on certain metallic surfaces, electrons are emitted from the surface – This is called the photoelectric effect – The emitted electrons are called photoelectrons w The effect was first discovered by Hertz w The successful explanation of the effect was given by Einstein in 1905 – Received Nobel Prize in 1921 for paper on electromagnetic radiation, of which the photoelectric effect was a part General Physics

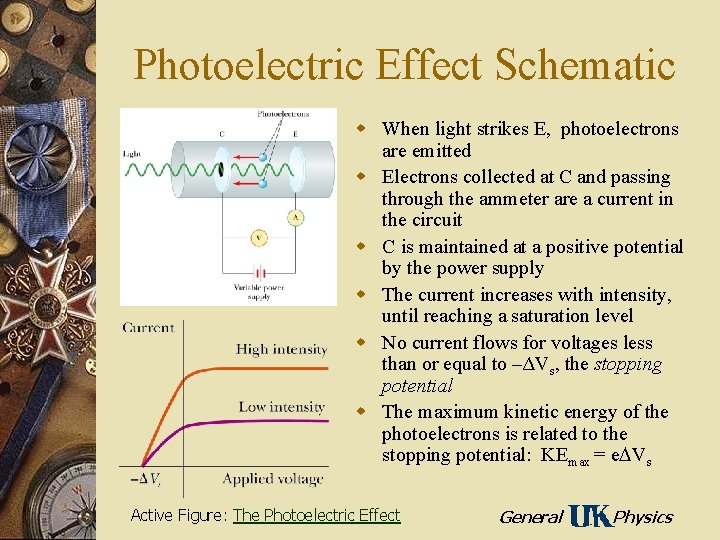
Photoelectric Effect Schematic w When light strikes E, photoelectrons are emitted w Electrons collected at C and passing through the ammeter are a current in the circuit w C is maintained at a positive potential by the power supply w The current increases with intensity, until reaching a saturation level w No current flows for voltages less than or equal to –ΔVs, the stopping potential w The maximum kinetic energy of the photoelectrons is related to the stopping potential: KEmax = e Vs Active Figure: The Photoelectric Effect General Physics

Features Not Explained by Classical Physics/Wave Theory w The stopping potential Vs (maximum kinetic energy KEmax) is independent of the radiation intensity w Instead, the maximum kinetic energy KEmax of the photoelectrons depends on the light frequency w No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated w Electrons are emitted from the surface almost instantaneously, even at very low intensities General Physics

Einstein’s Explanation w A tiny wave packet of light energy, called a photon, would be emitted when a quantized oscillator jumped from one energy level to the next lower one – Extended Planck’s idea of quantization to E. M. radiation w The photon’s energy would be E = hƒ w Each photon can give all its energy to an electron in the metal w The maximum kinetic energy of the liberated photoelectron is KEmax = hƒ – f w f is called the work function of the metal – it is the energy needed by the electron to escape the metal General Physics

Explains Classical “Problems” w KEmax = hƒ – f – The effect is not observed below a certain cutoff frequency since the photon energy must be greater than or equal to the work function – without enough energy, electrons are not emitted, regardless of the intensity of the light – The maximum KE depends only on the frequency and the work function, not on the intensity – The maximum KE increases with increasing frequency – The effect is instantaneous since there is a one-toone interaction between the photon and the electron General Physics

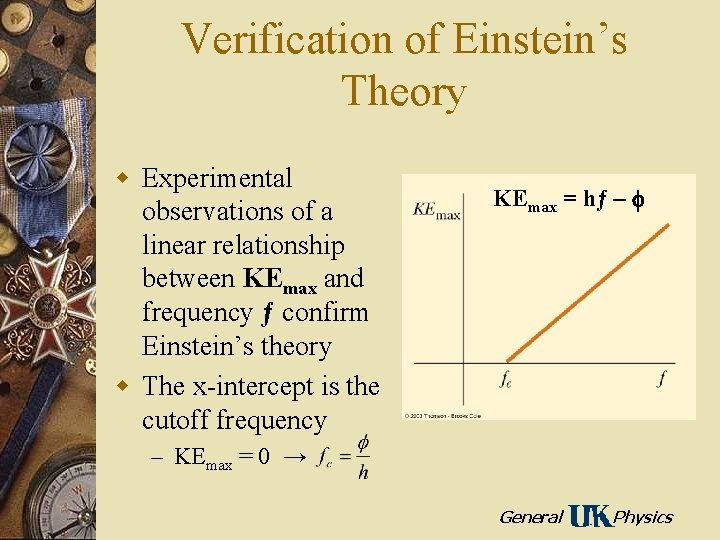
Verification of Einstein’s Theory w Experimental observations of a linear relationship between KEmax and frequency ƒ confirm Einstein’s theory w The x-intercept is the cutoff frequency KEmax = hƒ – f – KEmax = 0 → General Physics

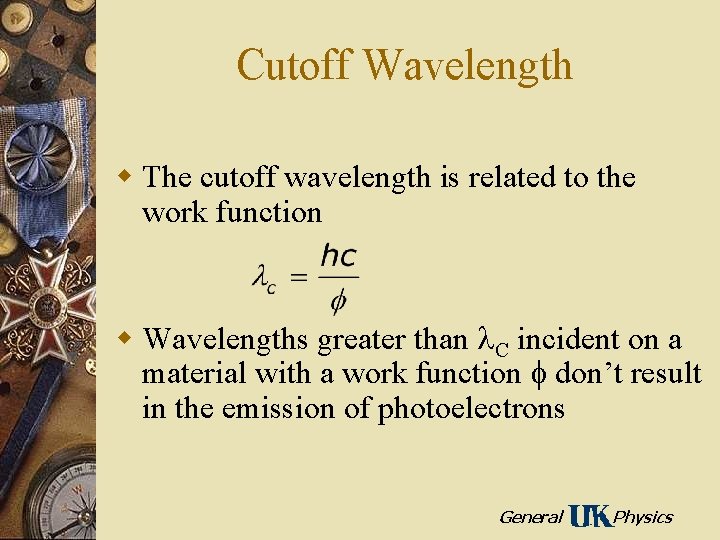
Cutoff Wavelength w The cutoff wavelength is related to the work function w Wavelengths greater than C incident on a material with a work function f don’t result in the emission of photoelectrons General Physics

Photocells w Photocells are an application of the photoelectric effect w When light of sufficiently high frequency falls on the cell, a current is produced w Examples – Streetlights, garage door openers, elevators General Physics

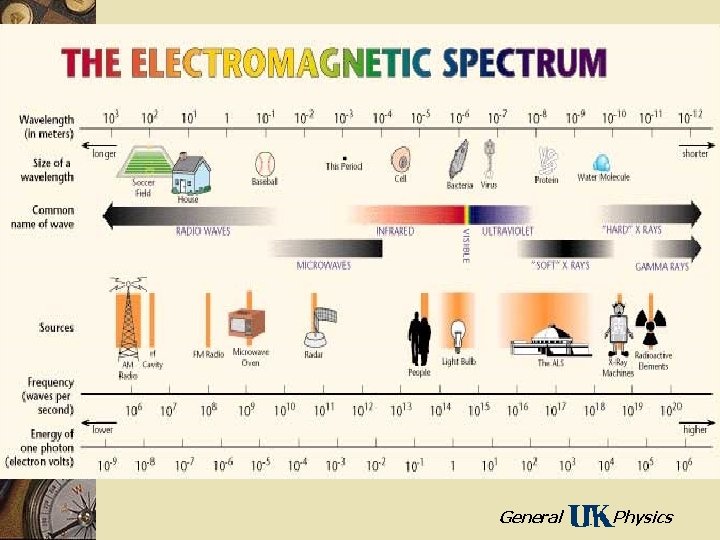
X-Rays w Electromagnetic radiation with short wavelengths – Wavelengths less than for ultraviolet – Wavelengths are typically about 0. 1 nm – X-rays have the ability to penetrate most materials with relative ease – High energy photons which can break chemical bonds – danger to tissue w Discovered and named by Roentgen in 1895 General Physics

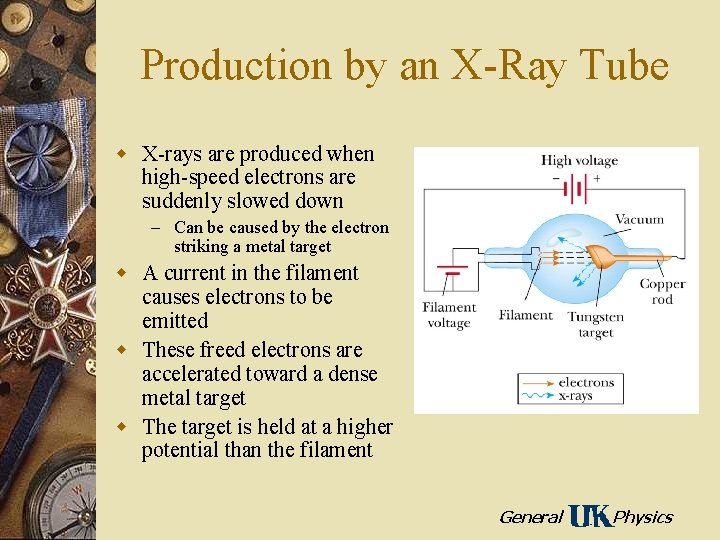
Production by an X-Ray Tube w X-rays are produced when high-speed electrons are suddenly slowed down – Can be caused by the electron striking a metal target w A current in the filament causes electrons to be emitted w These freed electrons are accelerated toward a dense metal target w The target is held at a higher potential than the filament General Physics

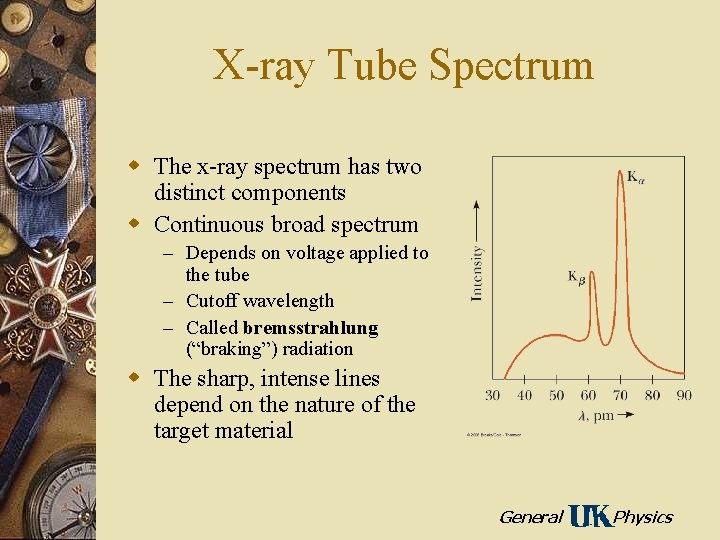
X-ray Tube Spectrum w The x-ray spectrum has two distinct components w Continuous broad spectrum – Depends on voltage applied to the tube – Cutoff wavelength – Called bremsstrahlung (“braking”) radiation w The sharp, intense lines depend on the nature of the target material General Physics

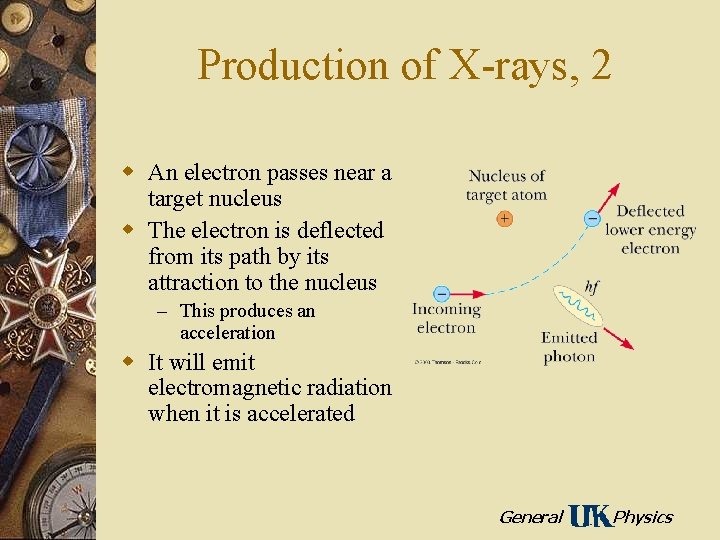
Production of X-rays, 2 w An electron passes near a target nucleus w The electron is deflected from its path by its attraction to the nucleus – This produces an acceleration w It will emit electromagnetic radiation when it is accelerated General Physics

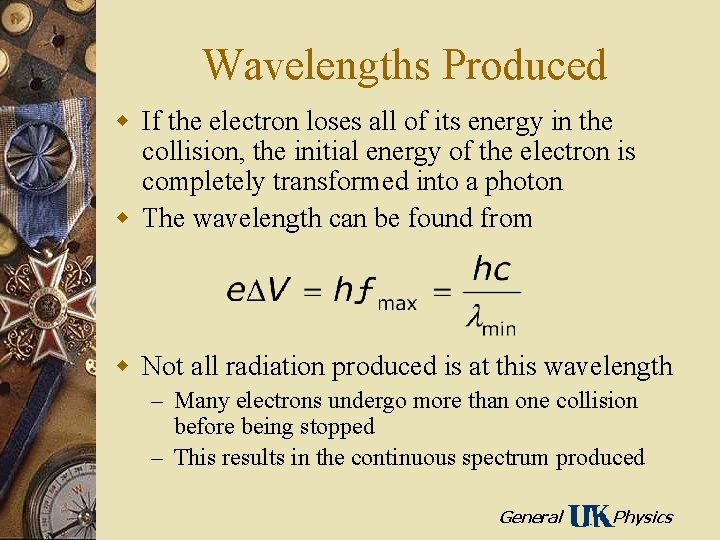
Wavelengths Produced w If the electron loses all of its energy in the collision, the initial energy of the electron is completely transformed into a photon w The wavelength can be found from w Not all radiation produced is at this wavelength – Many electrons undergo more than one collision before being stopped – This results in the continuous spectrum produced General Physics

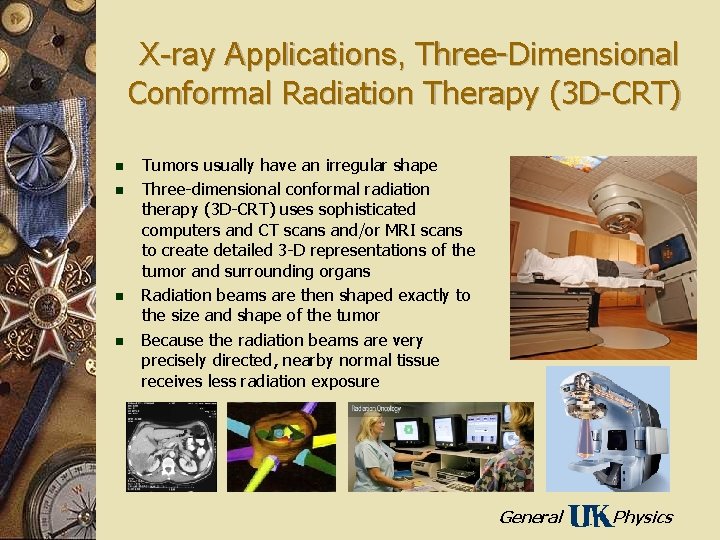
X-ray Applications, Three-Dimensional Conformal Radiation Therapy (3 D-CRT) n n Tumors usually have an irregular shape Three-dimensional conformal radiation therapy (3 D-CRT) uses sophisticated computers and CT scans and/or MRI scans to create detailed 3 -D representations of the tumor and surrounding organs Radiation beams are then shaped exactly to the size and shape of the tumor Because the radiation beams are very precisely directed, nearby normal tissue receives less radiation exposure General Physics