Chapter 10 1 How is energy released in
















- Slides: 37

Chapter 10

1. How is energy released in an explosion? a) through gaseous products b) thermal energy c) kinetic energy of flying debris d) all of the above

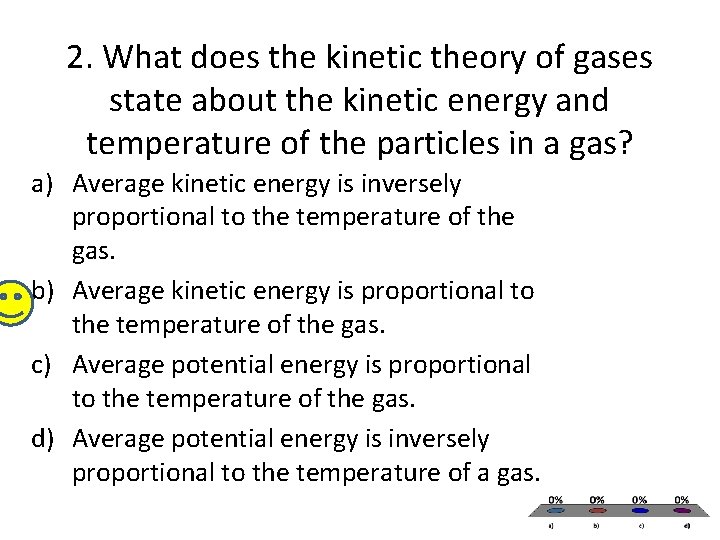
2. What does the kinetic theory of gases state about the kinetic energy and temperature of the particles in a gas? a) Average kinetic energy is inversely proportional to the temperature of the gas. b) Average kinetic energy is proportional to the temperature of the gas. c) Average potential energy is proportional to the temperature of the gas. d) Average potential energy is inversely proportional to the temperature of a gas.

3. Gas particles are extremely small and have relatively large distances between them. a) True b) False

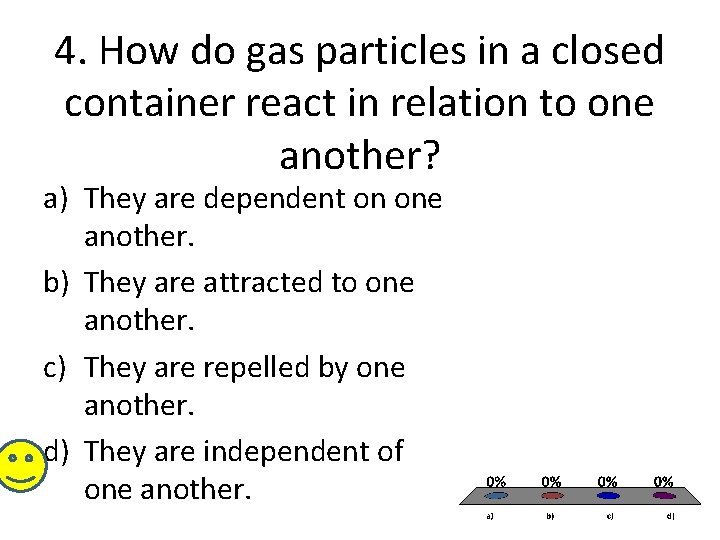
4. How do gas particles in a closed container react in relation to one another? a) They are dependent on one another. b) They are attracted to one another. c) They are repelled by one another. d) They are independent of one another.

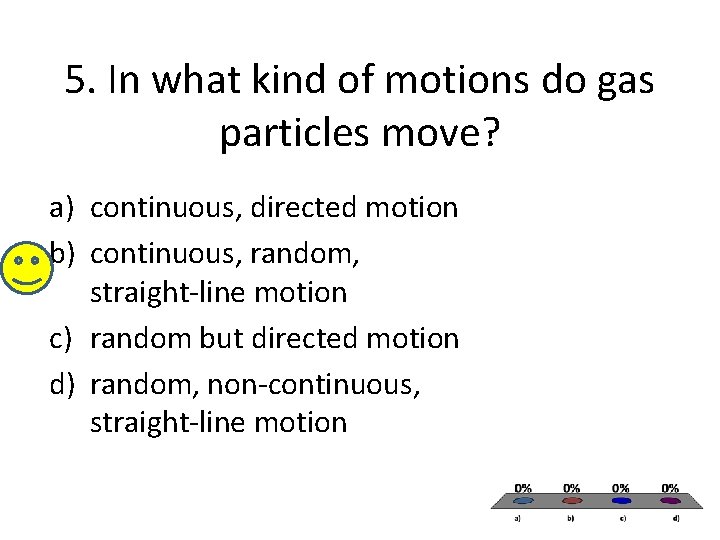
5. In what kind of motions do gas particles move? a) continuous, directed motion b) continuous, random, straight-line motion c) random but directed motion d) random, non-continuous, straight-line motion

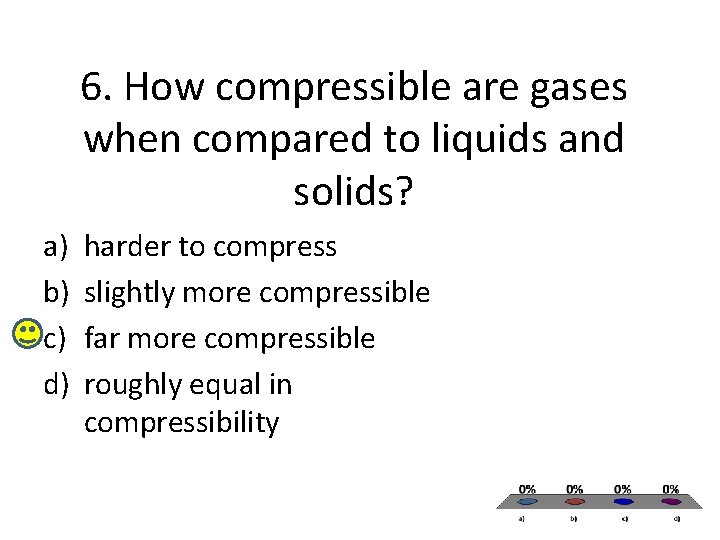
6. How compressible are gases when compared to liquids and solids? a) b) c) d) harder to compress slightly more compressible far more compressible roughly equal in compressibility

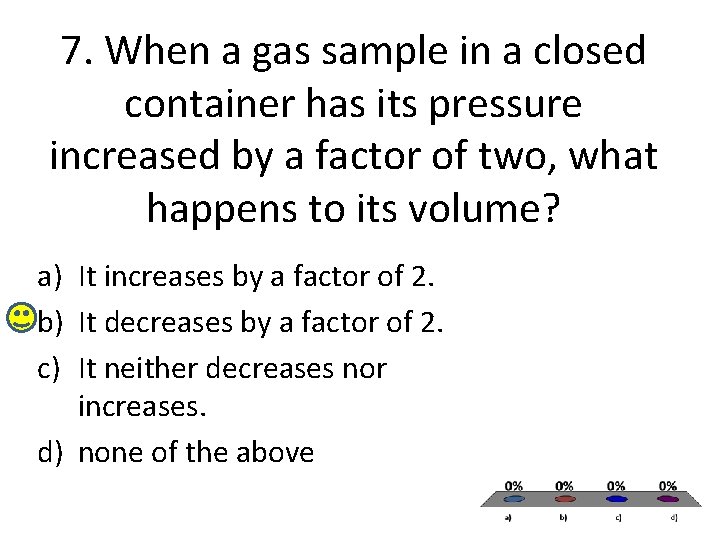
7. When a gas sample in a closed container has its pressure increased by a factor of two, what happens to its volume? a) It increases by a factor of 2. b) It decreases by a factor of 2. c) It neither decreases nor increases. d) none of the above

8. Which of the following is the best statement of Boyle's Law? a) The pressure of a gas is directly proportional to its volume. b) The pressure of a gas is inversely proportional to its volume. c) The volume of a gas is directly proportional to its temperature. d) The volume of a gas is inversely proportional to its temperature.

9. A 4 -L container of gas at 760 torr is compressed to 2. 0 L. What is its new pressure? a) b) c) d) 7, 600 torr 1, 216 torr 1520 torr 380 torr

10. A 2 -L container of gas at 1, 060 torr is expanded to 4. 0 L. What is its new pressure? a) b) c) d) 326 torr 2, 120 torr 3, 445 torr 530 torr

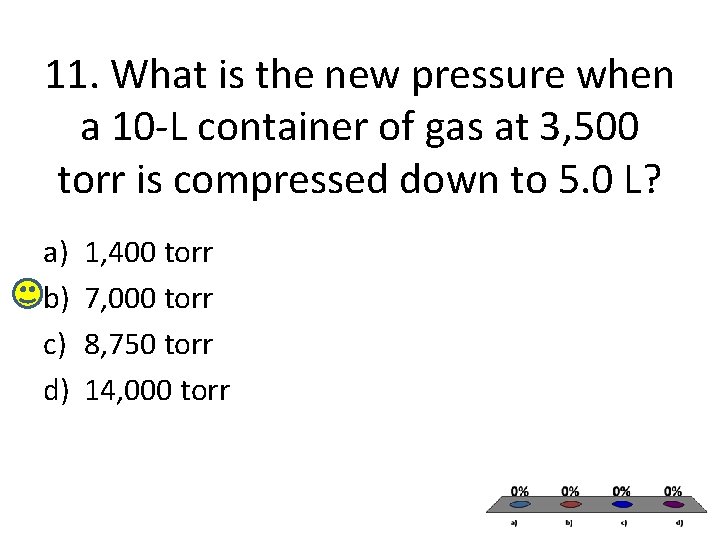
11. What is the new pressure when a 10 -L container of gas at 3, 500 torr is compressed down to 5. 0 L? a) b) c) d) 1, 400 torr 7, 000 torr 8, 750 torr 14, 000 torr

12. Of the following, which is the best expression of Gay-Lussac's Law? a) The temperature of a gas is inversely related to its pressure. b) The pressure of a gas can be directly related to its volume. c) The pressure of a gas is directly related to its temperature, when all other factors are equal. d) The pressure of a gas is inversely related to its temperature when all other factors are equal.

13. When the temperature of a gas rises by a factor of 6, what should happen to the pressure of the gas, all other factors being constant? a) It should rise by a factor of 6. b) It should decrease by a factor of 6. c) The pressure should remain unchanged. d) not enough information to tell

14. What happens to the pressure of a gas when the temperature rises if there are no other factors that change? a) b) c) d) nothing It rises as well. It decreases. none of the above

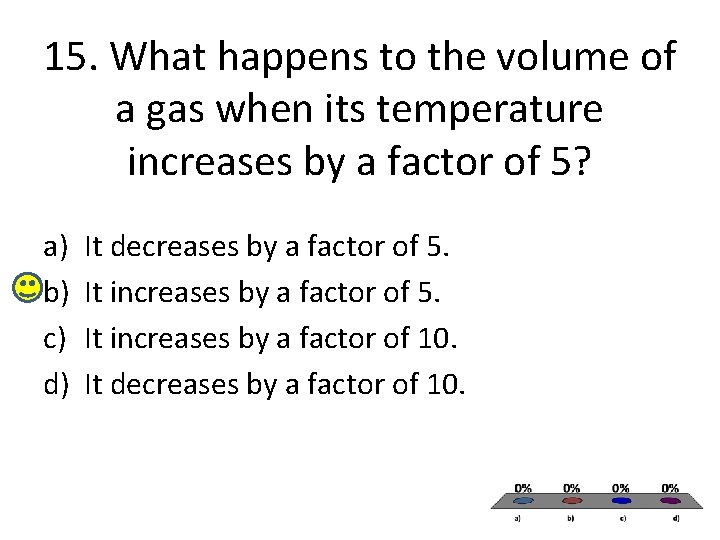
15. What happens to the volume of a gas when its temperature increases by a factor of 5? a) b) c) d) It decreases by a factor of 5. It increases by a factor of 10. It decreases by a factor of 10.

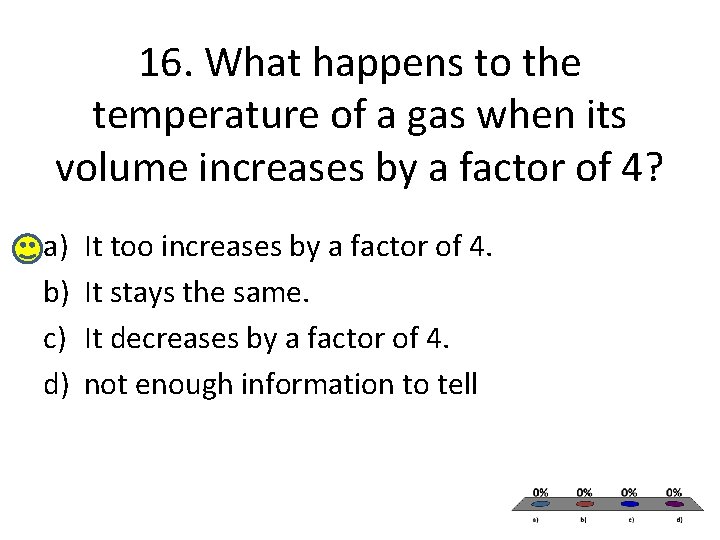
16. What happens to the temperature of a gas when its volume increases by a factor of 4? a) b) c) d) It too increases by a factor of 4. It stays the same. It decreases by a factor of 4. not enough information to tell

17. What happens to the volume of a gas when its temperature doubles? a) nothing b) It increases to twice the original. c) It decreases to 1/2 of the original. d) not enough information to tell

18. What happens to the temperature of a gas when its volume increases by a factor of 5, and its pressure changes? a) b) c) d) It increases by a factor of 5. It decreases by a factor of 5. It increases by a factor of 2. 5. not enough information to tell

19. What happens to the pressure of a gas when its volume decreases, all other factors being equal? a) b) c) d) The pressure increases. The pressure decreases. The pressure remains equal. B or C

20. With all other factors remaining constant, how does the temperature of a gas shift when the pressure increases? a) b) c) d) It remains constant. It decreases. It increases. B or C, depending on how great the shift is

21. When the volume of a gas decreases, what happens to its temperature if all other factors remain unchanged? a) b) c) d) It remains constant. It increases. It decreases. A pressure value is also needed to answer this.

22. If 12. 0 atm of nitrogen gas in a 16. 0 L container is compressed to 4. 0 L, what is the resulting pressure? a) b) c) d) 48. 0 atm 6. 0 atm 3. 5 atm 3. 0 atm

23. If a 20. 0 L container of oxygen has a pressure of 15. 0 atm, what will the volume be if the pressure is changed to 10. 0 atm? a) b) c) d) 10. 0 L 40. 0 L 15. 0 L 30. 0 L

24. If a gas system at 25 o. C has a pressure of 25. 0 atm, what pressure will the system change to o if the temperature rises to 35 C? a) b) c) d) 38. 0 atm 30. 5 atm 25. 8 atm 16. 4 atm

25. If a gas at a pressure of 15. 0 atm has a temperature of 300 K, what will its temperature be if the pressure changes to 30. 0 atm? a) b) c) d) 900 K 100 K 390 K 600 K

26. What volume will a gas occupy if at 298 K and 150 L its temperature is changed to 308 K? a) b) c) d) 155 L 140 L 103 L 30. 0 L

27. 5. 00 L of chlorine gas is at 288 K. What temperature will the gas have when the volume changes to 12. 0 L? a) b) c) d) 691 K 115 K 720 K 300 K

28. If a gas at 10 atm occupying 5. 0 L at 25 o. C is pressurized to 25 atm at 10 L, what will the new temperature of the gas be? a) b) c) d) 600 K 933 K 540 K 1490 K

29. When a gas, starting at 20 atm and 4. 0 L at 293 K, is changed to 35 atm and 303 K, what is the new volume? a) b) c) d) 3. 0 L 2. 4 L 6. 0 L 1. 2 L
30. If a gas at 10 L and 15. 0 atm is at 293 K, when the pressure changes to 30. 0 atm and the temperature drops to 283 K, what is the new volume? a) b) c) d) 8. 33 L 6. 44 L 4. 82 L 7, 500 L

31. At an altitude of 4000 m, the air pressure is approximately 0. 30 atm. Most airplane cabins are pressurized to 0. 60 atm. If the pressurized cabin of an airplane is breached at an altitude of 4000 m, use Boyle’s law to determine what will happen to the volume of air in the lungs of an airplane passenger? a) The volume of air in the passenger’s lungs will double. b) The volume of the air in the passenger’s lungs will decrease by half. c) The volume of the passenger’s lungs will stay the same. d) There is not enough information to understand what would happen to the passenger’s lungs.

32. Why do the manufacturers of automobile tires recommend that tire pressures be measured several times per year? a) The pressure of air in the tires changes with seasonal temperature variations. b) During the winter months, it is necessary to add air tothe tires to maintain the proper tire pressure. c) As the weather warms up, the air pressure in the tire increases and the excess pressure must be released. d) All the above are true because the pressure of the gas is directly related to the temperature.

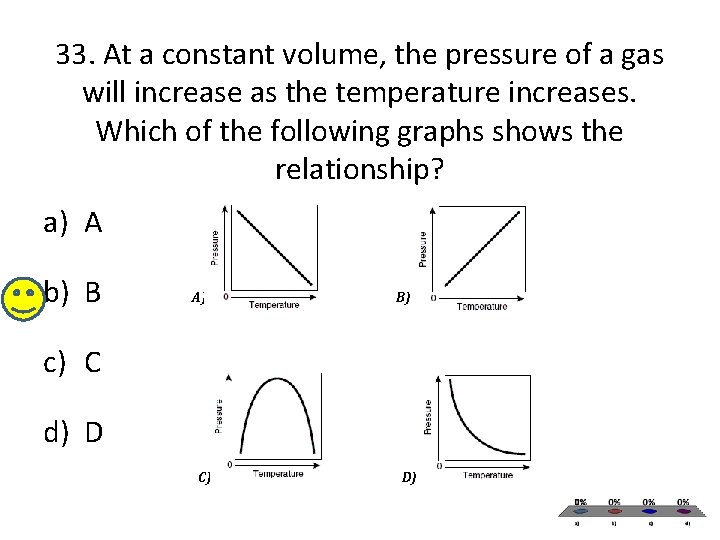
33. At a constant volume, the pressure of a gas will increase as the temperature increases. Which of the following graphs shows the relationship? a) A b) B A) B) c) C d) D C) D)

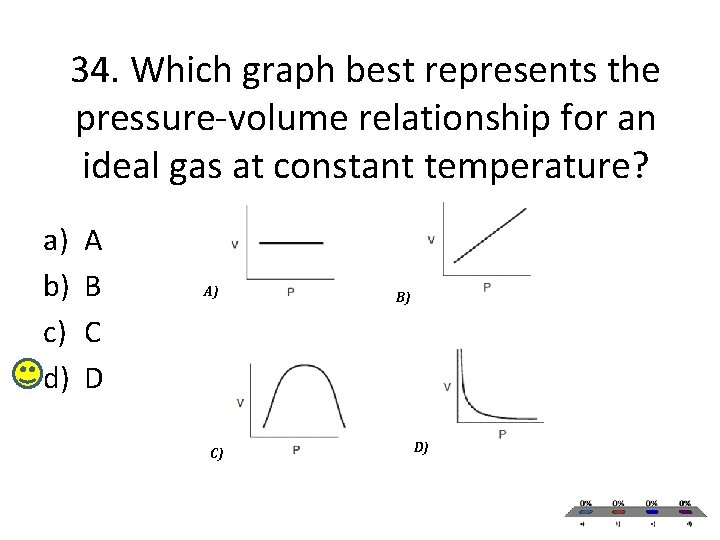
34. Which graph best represents the pressure-volume relationship for an ideal gas at constant temperature? a) b) c) d) A B C D A) C) B) D)

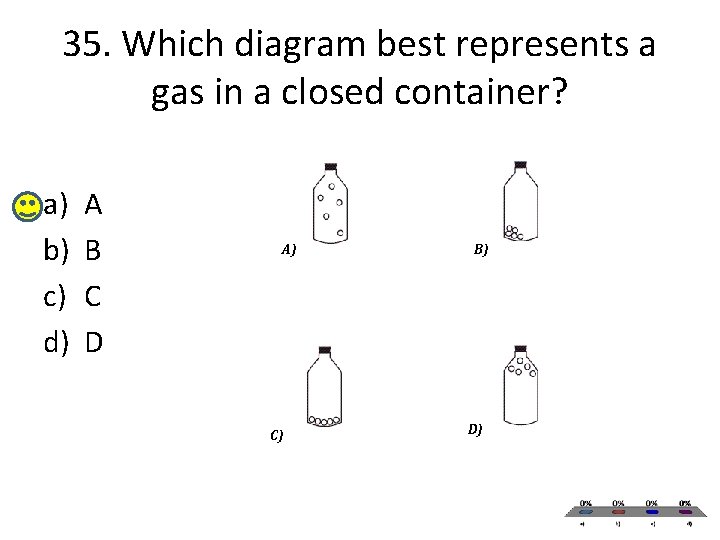
35. Which diagram best represents a gas in a closed container? a) b) c) d) A B C D A) C) B) D)

• • • Practice calculating: Boyles’ Law P 1 V 1 = P 2 V 2 Charles’ Law T 1 V 2 = T 2 V 1 G-L’s Law P 1 T 2 = P 2 T 1 Combined Gas Law P 1 V 1 T 2= P 2 V 2 T 1