Voltaic GalvanicCells The energy released in a spontaneous












- Slides: 18

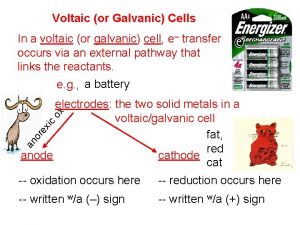
Voltaic (Galvanic)Cells • The energy released in a spontaneous redox reaction is used to perform electrical work. • Voltaic or galvanic cells are devices in which electron transfer occurs via an external circuit. • Voltaic cells are spontaneous. • If a strip of Zn is placed in a solution of Cu. SO 4, Cu is deposited on the Zn and the Zn dissolves by forming Zn 2+.

• Zn is spontaneously oxidized to Zn 2+ by Cu 2+. • The Cu 2+ is spontaneously reduced to Cu 0 by Zn. • The entire process is spontaneous.


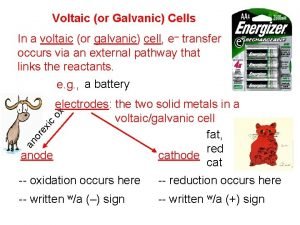
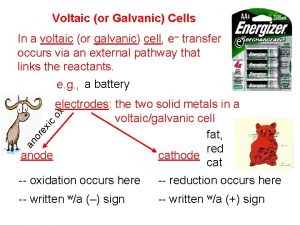
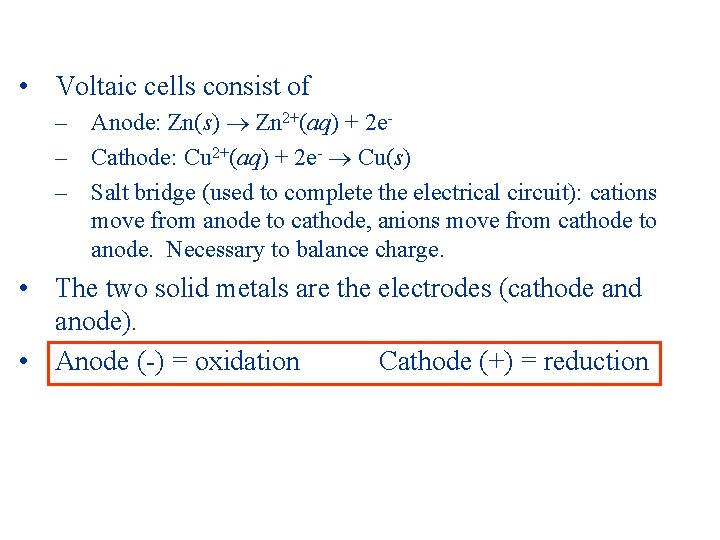
• Voltaic cells consist of – Anode: Zn(s) Zn 2+(aq) + 2 e– Cathode: Cu 2+(aq) + 2 e- Cu(s) – Salt bridge (used to complete the electrical circuit): cations move from anode to cathode, anions move from cathode to anode. Necessary to balance charge. • The two solid metals are the electrodes (cathode and anode). • Anode (-) = oxidation Cathode (+) = reduction

• As oxidation occurs, Zn is converted to Zn 2+ and 2 e-. The electrons flow towards the cathode (through the wire) where they are used in the reduction reaction. • We expect the Zn electrode to lose mass and the Cu electrode to gain mass. • “Rules” of voltaic cells: 1. At the anode electrons are products. (Oxidation) 2. At the cathode electrons are reactants. (Reduction) 3. Electrons cannot swim.

• Electrons flow from the anode to the cathode. • Therefore, the anode is negative and the cathode is positive. • Electrons cannot flow through the solution; they have to be transported through an external wire. (Rule 3. )


• Anions and cations move through a porous barrier or salt bridge. • Cations move into the cathodic compartment to neutralize the excess negatively charged ions (Cathode: Cu 2+ + 2 e- Cu, so the counterion of Cu is in excess). • Anions move into the anodic compartment to neutralize the excess Zn 2+ ions formed by oxidation.

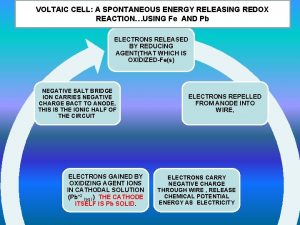
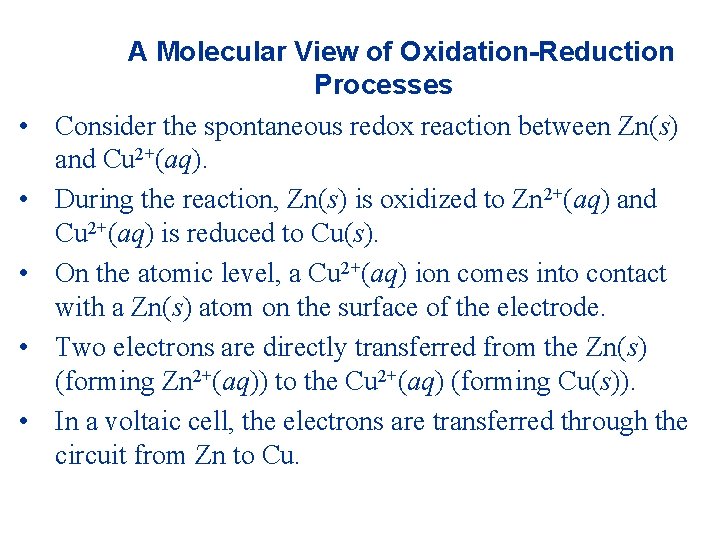
• • • A Molecular View of Oxidation-Reduction Processes Consider the spontaneous redox reaction between Zn(s) and Cu 2+(aq). During the reaction, Zn(s) is oxidized to Zn 2+(aq) and Cu 2+(aq) is reduced to Cu(s). On the atomic level, a Cu 2+(aq) ion comes into contact with a Zn(s) atom on the surface of the electrode. Two electrons are directly transferred from the Zn(s) (forming Zn 2+(aq)) to the Cu 2+(aq) (forming Cu(s)). In a voltaic cell, the electrons are transferred through the circuit from Zn to Cu.



Cell EMF • The flow of electrons from anode to cathode is spontaneous. • Electrons flow from anode to cathode because the cathode has a lower electrical potential energy than the anode. • Potential difference: difference in electrical potential. Measured in volts. • One volt is the potential difference required to impart one joule of energy to a charge of one coulomb:

• Electromotive force (emf) is the force required to push electrons through the external circuit. • Cell potential: Ecell is the emf of a cell. • For 1 M solutions at 25 C (standard conditions), the standard emf (standard cell potential) is called E cell.

Standard Reduction (Half-Cell) Potentials • Convenient tabulation of electrochemical data. • Standard reduction potentials, E red are measured relative to the standard hydrogen electrode (SHE). • Oxidation Potentials are obtained by reversing a reduction half-reaction (sign of E° is changed)



• The SHE is the cathode. It consists of a Pt electrode in a tube placed in 1 M H+ solution. H 2 is bubbled through the tube. • For the SHE, we assign 2 H+(aq, 1 M) + 2 e- H 2(g, 1 atm) • E red of zero. • The emf of a cell can be calculated from standard reduction potentials:

 Balance redox
Balance redox Voltaic cell electron flow
Voltaic cell electron flow Voltaic cells example #2 worksheet answers
Voltaic cells example #2 worksheet answers Primary voltaic cell
Primary voltaic cell Galvanic vs electrolytic cell
Galvanic vs electrolytic cell Voltaic cell virtual lab
Voltaic cell virtual lab Voltaic fundamental
Voltaic fundamental Voltaic fundamentals
Voltaic fundamentals Voltaic fundamentals
Voltaic fundamentals Voltaic and galvanic cells
Voltaic and galvanic cells Oxidation state
Oxidation state Voltaic aim
Voltaic aim Gibbs free energy of formation
Gibbs free energy of formation Gibbs energy and equilibrium
Gibbs energy and equilibrium Relationship between entropy and free energy
Relationship between entropy and free energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Gradecscope
Gradecscope Staar released test
Staar released test