Physiology of vasopressin secretion THE antidiuretic hormone ADH






- Slides: 32

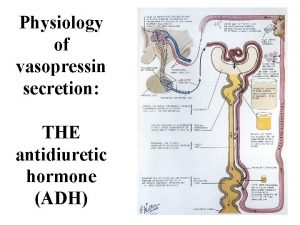
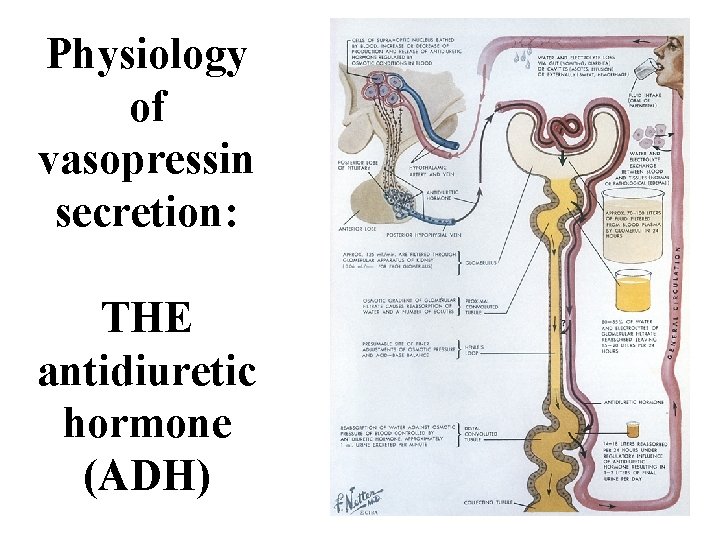
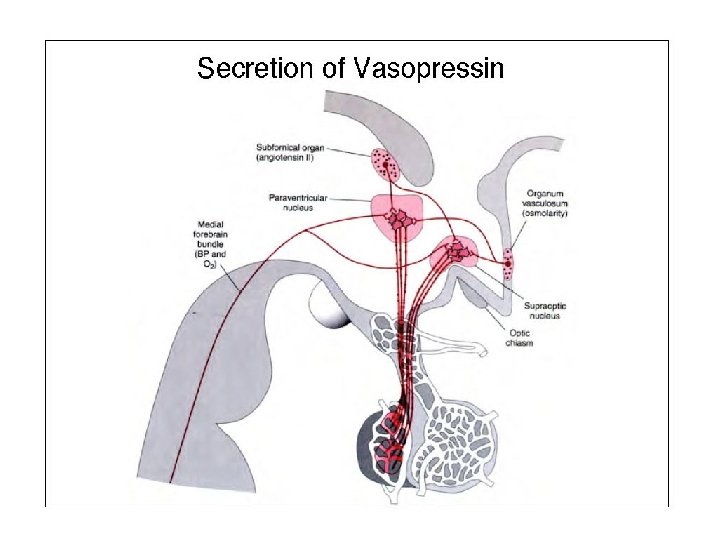
Physiology of vasopressin secretion: THE antidiuretic hormone (ADH)

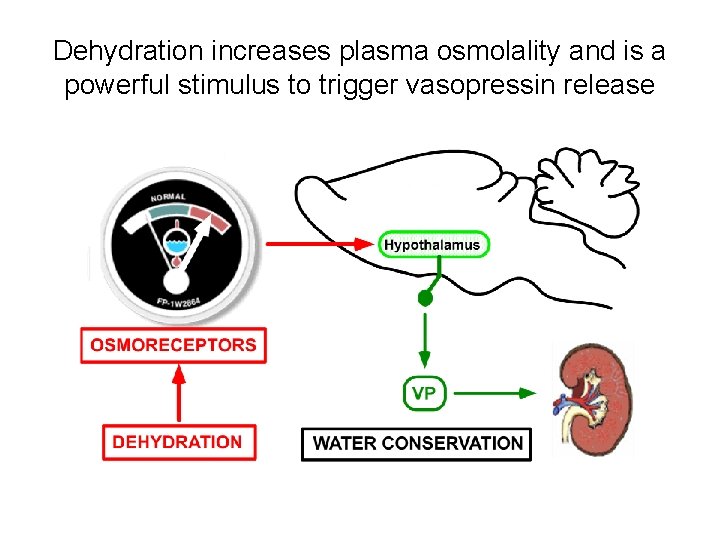
Secretion of ADH • The biological action of ADH is to conserve body water and regulate tonicity of body fluids. • It is primarily regulated by osmotic and volume stimuli. • Water deprivation increases osmolality of plasma which activates hypothalmic osmoreceptors to stimulate ADH release.


Dehydration increases plasma osmolality and is a powerful stimulus to trigger vasopressin release

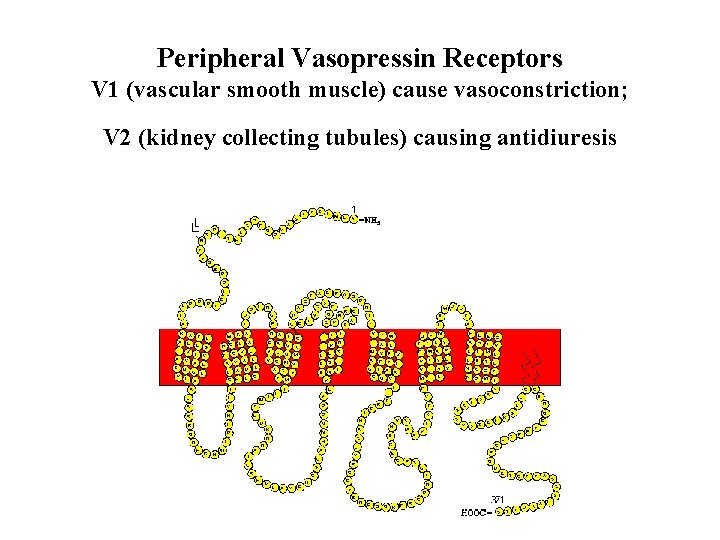
Peripheral Vasopressin Receptors V 1 (vascular smooth muscle) cause vasoconstriction; V 2 (kidney collecting tubules) causing antidiuresis

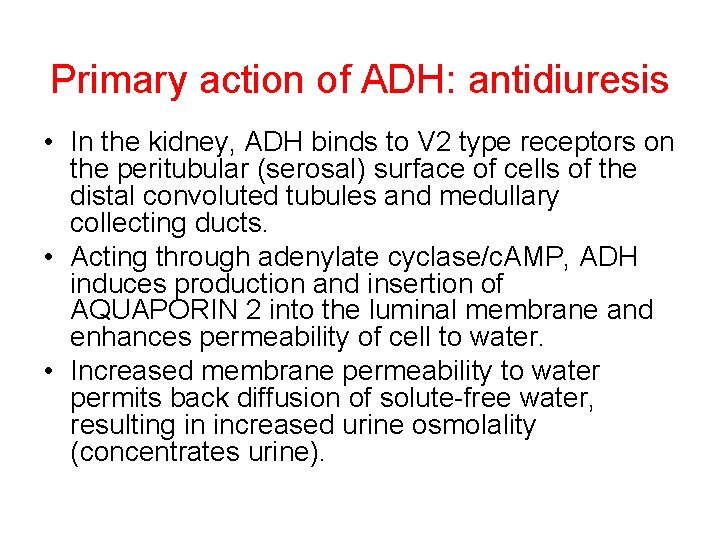
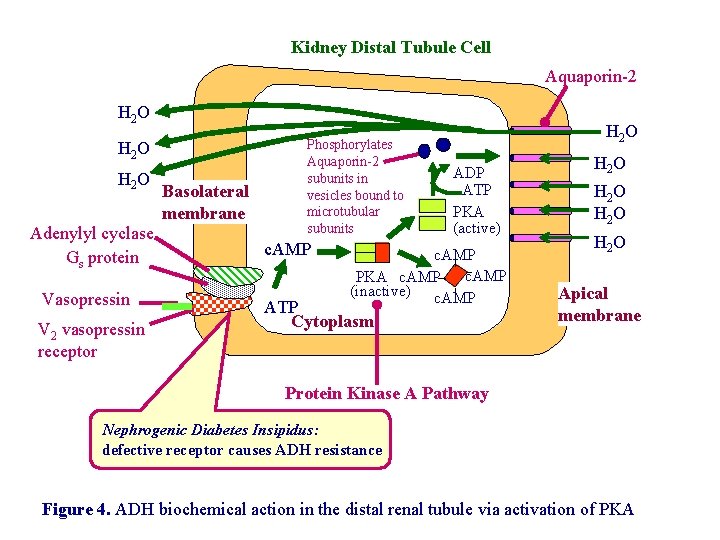
Primary action of ADH: antidiuresis • In the kidney, ADH binds to V 2 type receptors on the peritubular (serosal) surface of cells of the distal convoluted tubules and medullary collecting ducts. • Acting through adenylate cyclase/c. AMP, ADH induces production and insertion of AQUAPORIN 2 into the luminal membrane and enhances permeability of cell to water. • Increased membrane permeability to water permits back diffusion of solute-free water, resulting in increased urine osmolality (concentrates urine).

Kidney Distal Tubule Cell Aquaporin-2 H 2 O Adenylyl cyclase Gs protein Vasopressin V 2 vasopressin receptor Basolateral membrane Phosphorylates Aquaporin-2 subunits in vesicles bound to microtubular subunits c. AMP H 2 O ADP ATP PKA (active) c. AMP PKA c. AMP (inactive) c. AMP ATP Cytoplasm H 2 O Apical membrane Protein Kinase A Pathway Nephrogenic Diabetes Insipidus: defective receptor causes ADH resistance Figure 4. ADH biochemical action in the distal renal tubule via activation of PKA

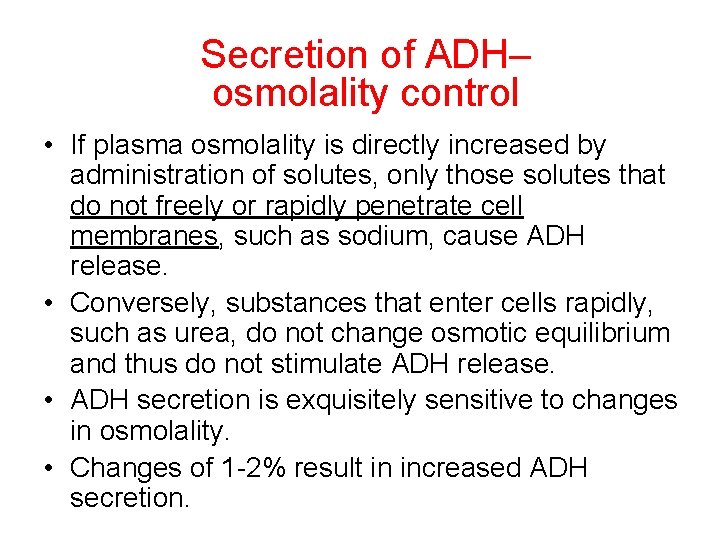
Secretion of ADH– osmolality control • If plasma osmolality is directly increased by administration of solutes, only those solutes that do not freely or rapidly penetrate cell membranes, such as sodium, cause ADH release. • Conversely, substances that enter cells rapidly, such as urea, do not change osmotic equilibrium and thus do not stimulate ADH release. • ADH secretion is exquisitely sensitive to changes in osmolality. • Changes of 1 -2% result in increased ADH secretion.

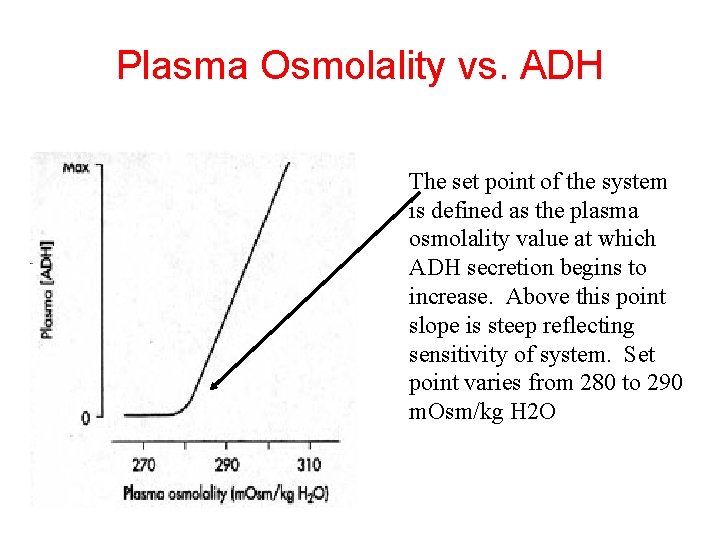
Plasma Osmolality vs. ADH The set point of the system is defined as the plasma osmolality value at which ADH secretion begins to increase. Above this point slope is steep reflecting sensitivity of system. Set point varies from 280 to 290 m. Osm/kg H 2 O

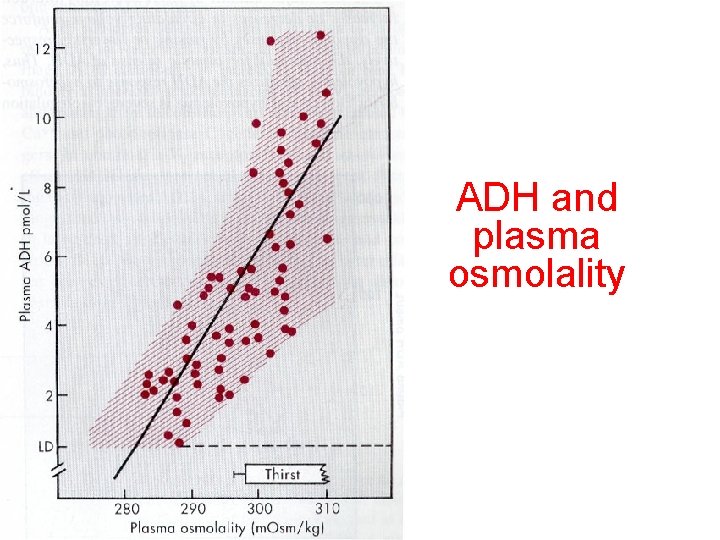
ADH and plasma osmolality

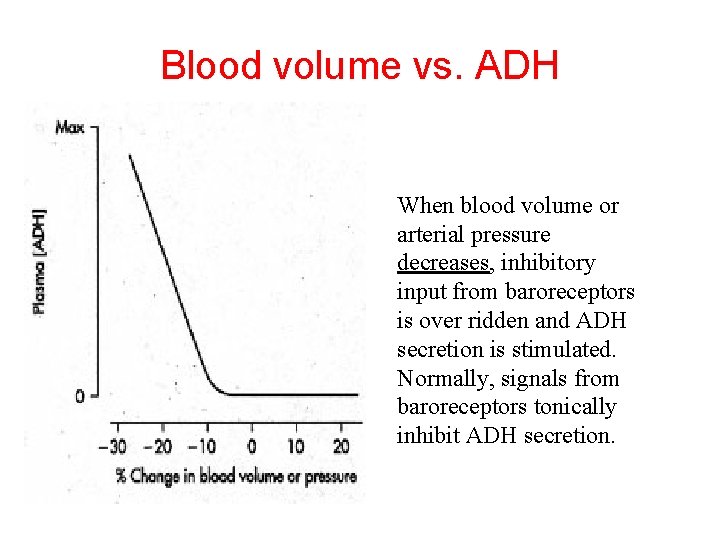
Blood volume vs. ADH When blood volume or arterial pressure decreases, inhibitory input from baroreceptors is over ridden and ADH secretion is stimulated. Normally, signals from baroreceptors tonically inhibit ADH secretion.

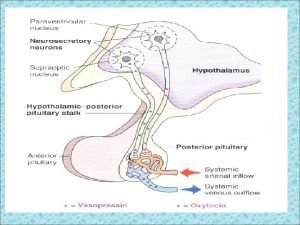
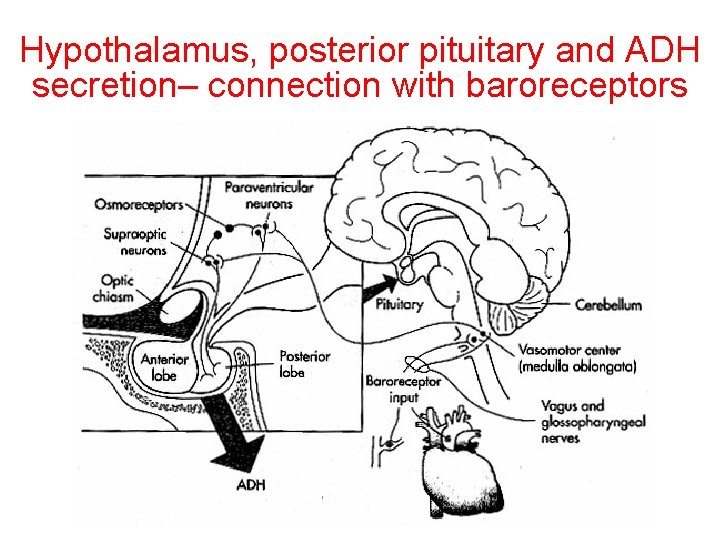
Hypothalamus, posterior pituitary and ADH secretion– connection with baroreceptors

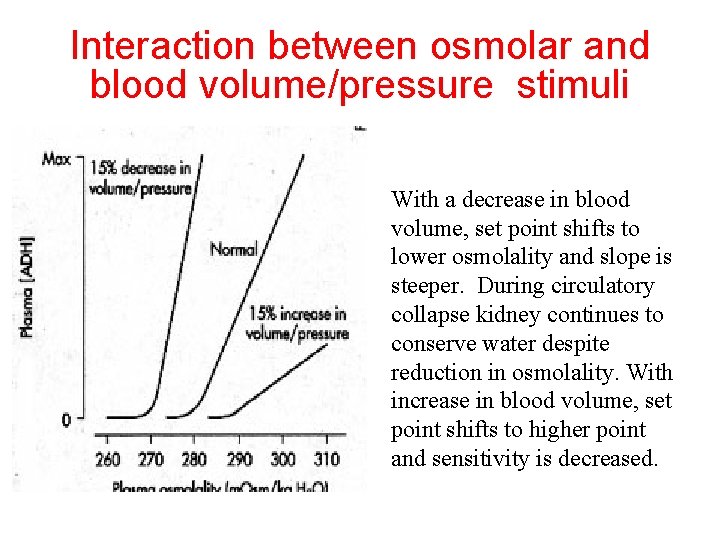
Interaction between osmolar and blood volume/pressure stimuli With a decrease in blood volume, set point shifts to lower osmolality and slope is steeper. During circulatory collapse kidney continues to conserve water despite reduction in osmolality. With increase in blood volume, set point shifts to higher point and sensitivity is decreased.

So what happens if you: -secrete too much ADH?

Syndrome of inappropriate ADH secretion • Features: inappropriately low plasma osmolality vs urine osmolality; low serum sodium, excessive kidney sodium excretion. • Causes: (multiple) e. g Carcinomas, CNS injury, postoperative… • Rx: restrict water intake, correct Na levels

So what happens if you: -Cannot make ADH? -Unable to secrete ADH? -Have non-functional receptors?

You have a condition known as Diabetes Insipidus • Polydipsia • Polyuria

Lack of vasopressin or VP receptor dysfunction results in a condition known as Diabetes Insipidus (DI) [also known as water diabetes, often mistaken for diabetes mellitus or sugar diabetes]. Causes: Neurogenic (central, hypothalamic, pituitary) Nephrogenic (vasopressin resistant) Gestagenic (gestational, during pregnancy) Dipsogenic (excessive water intake)

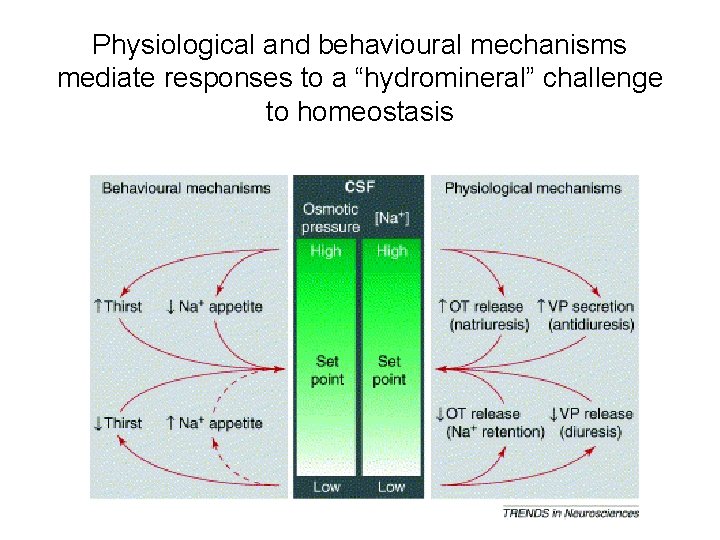
Physiological and behavioural mechanisms mediate responses to a “hydromineral” challenge to homeostasis

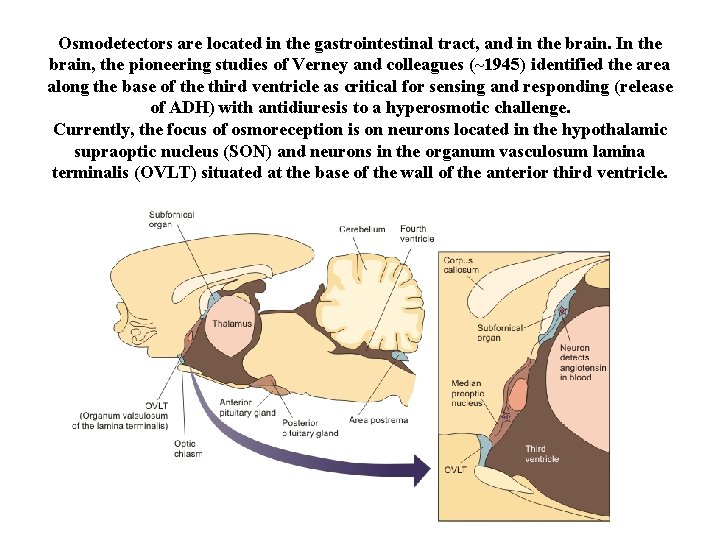
Osmodetectors are located in the gastrointestinal tract, and in the brain. In the brain, the pioneering studies of Verney and colleagues (~1945) identified the area along the base of the third ventricle as critical for sensing and responding (release of ADH) with antidiuresis to a hyperosmotic challenge. Currently, the focus of osmoreception is on neurons located in the hypothalamic supraoptic nucleus (SON) and neurons in the organum vasculosum lamina terminalis (OVLT) situated at the base of the wall of the anterior third ventricle.

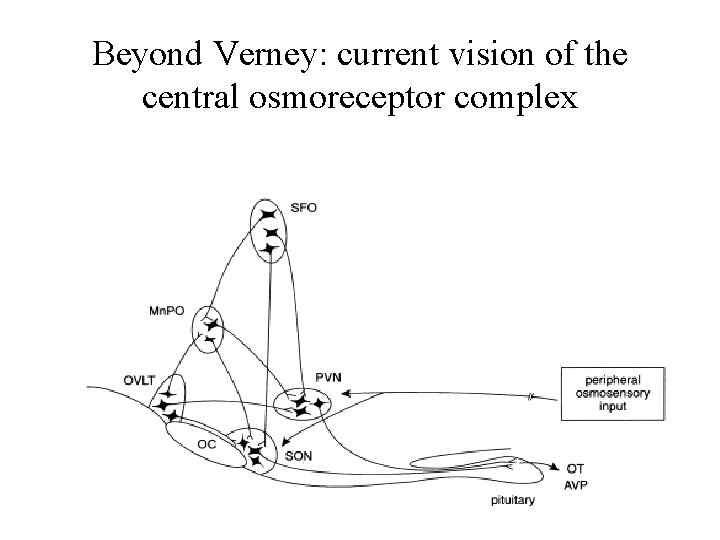
Beyond Verney: current vision of the central osmoreceptor complex

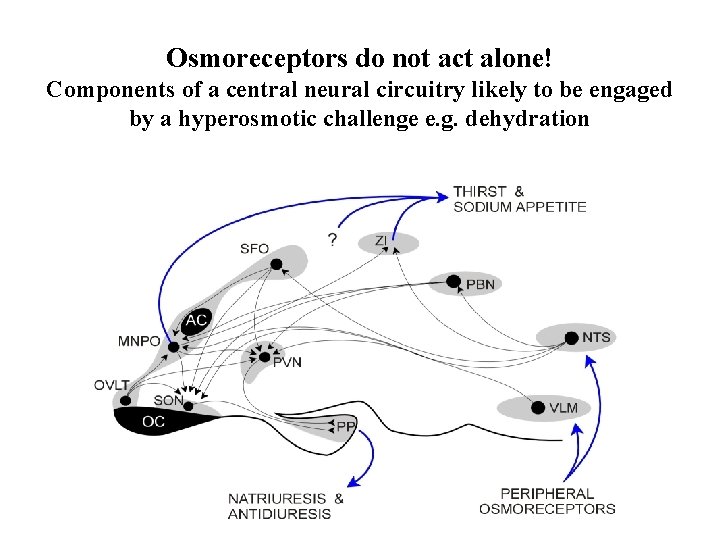
Osmoreceptors do not act alone! Components of a central neural circuitry likely to be engaged by a hyperosmotic challenge e. g. dehydration

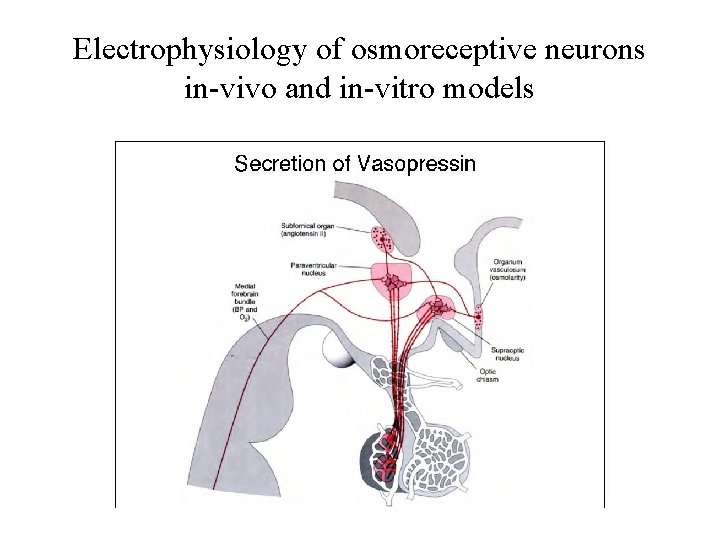
Electrophysiology of osmoreceptive neurons in-vivo and in-vitro models

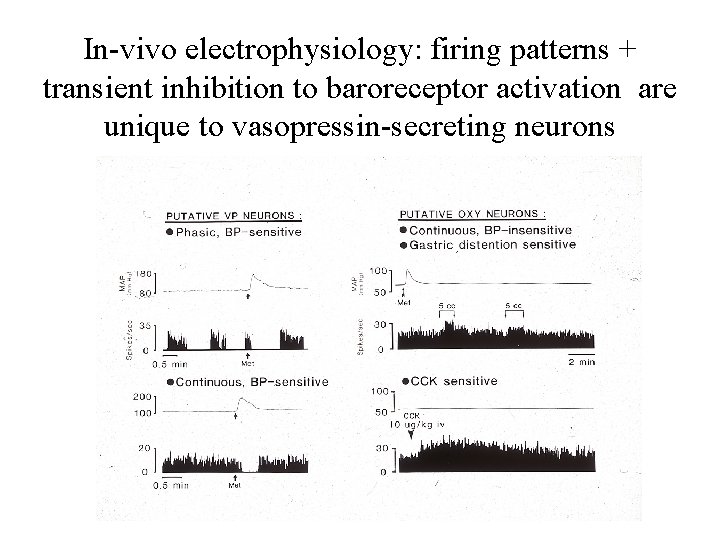
In-vivo electrophysiology: firing patterns + transient inhibition to baroreceptor activation are unique to vasopressin-secreting neurons

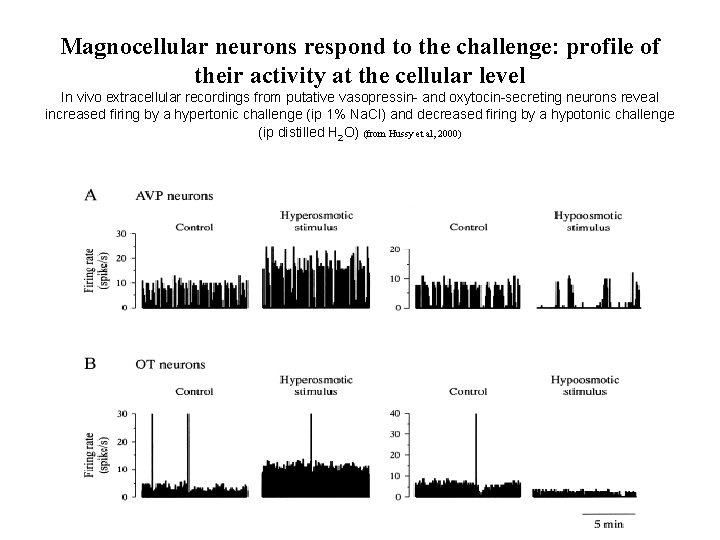
Magnocellular neurons respond to the challenge: profile of their activity at the cellular level In vivo extracellular recordings from putative vasopressin- and oxytocin-secreting neurons reveal increased firing by a hypertonic challenge (ip 1% Na. Cl) and decreased firing by a hypotonic challenge (ip distilled H 2 O) (from Hussy et al, 2000)

In-vitro electrophysiolgy: intracellular recordings in brain slices /dissociated cells

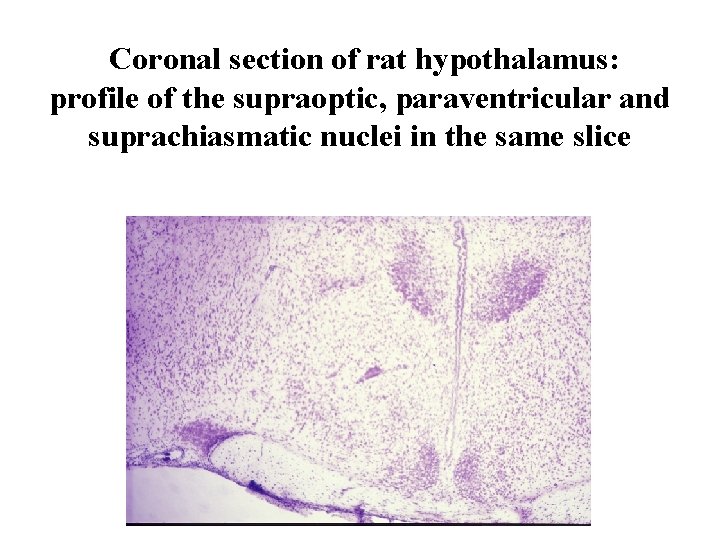
Coronal section of rat hypothalamus: profile of the supraoptic, paraventricular and suprachiasmatic nuclei in the same slice

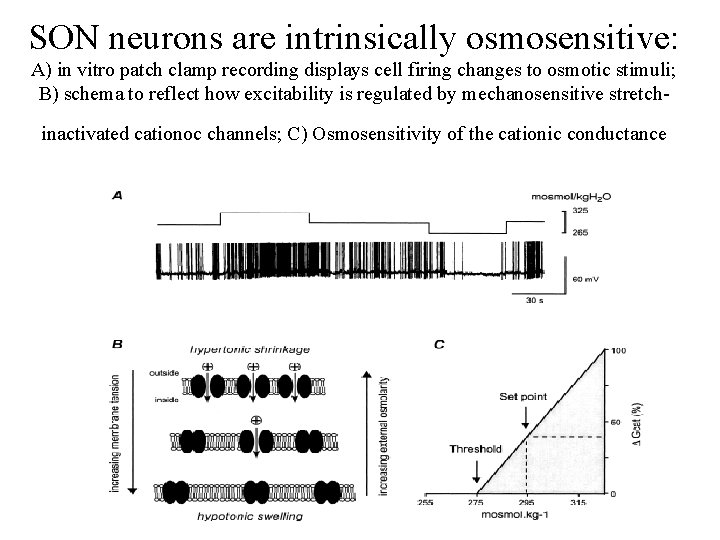
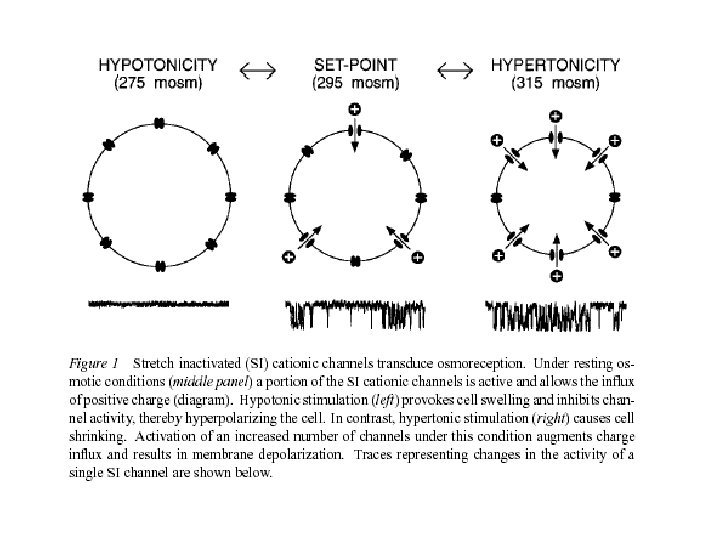
SON neurons are intrinsically osmosensitive: A) in vitro patch clamp recording displays cell firing changes to osmotic stimuli; B) schema to reflect how excitability is regulated by mechanosensitive stretchinactivated cationoc channels; C) Osmosensitivity of the cationic conductance


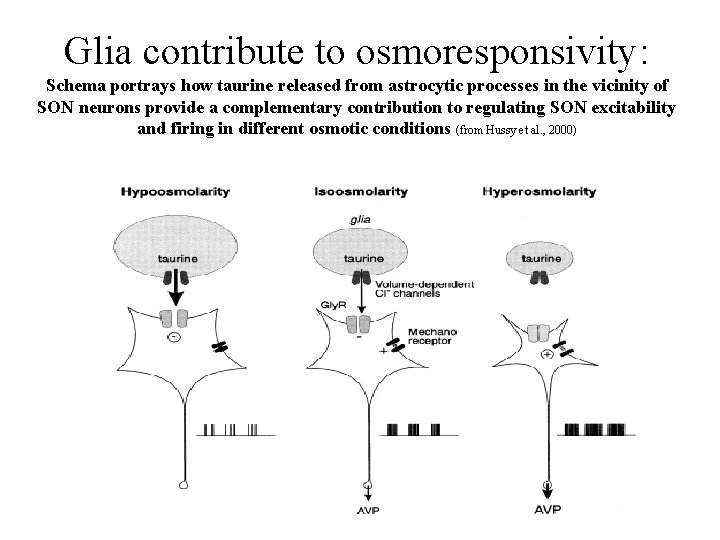
Glia contribute to osmoresponsivity: Schema portrays how taurine released from astrocytic processes in the vicinity of SON neurons provide a complementary contribution to regulating SON excitability and firing in different osmotic conditions (from Hussy et al. , 2000)

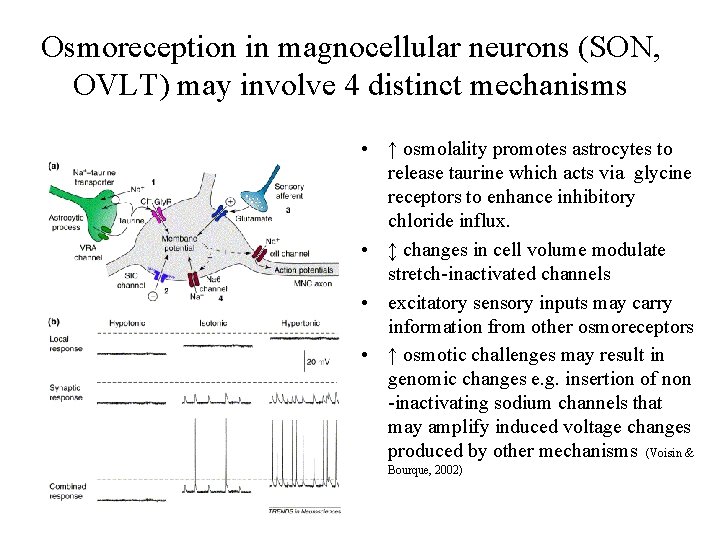
Osmoreception in magnocellular neurons (SON, OVLT) may involve 4 distinct mechanisms • ↑ osmolality promotes astrocytes to release taurine which acts via glycine receptors to enhance inhibitory chloride influx. • ↕ changes in cell volume modulate stretch-inactivated channels • excitatory sensory inputs may carry information from other osmoreceptors • ↑ osmotic challenges may result in genomic changes e. g. insertion of non -inactivating sodium channels that may amplify induced voltage changes produced by other mechanisms (Voisin & Bourque, 2002)

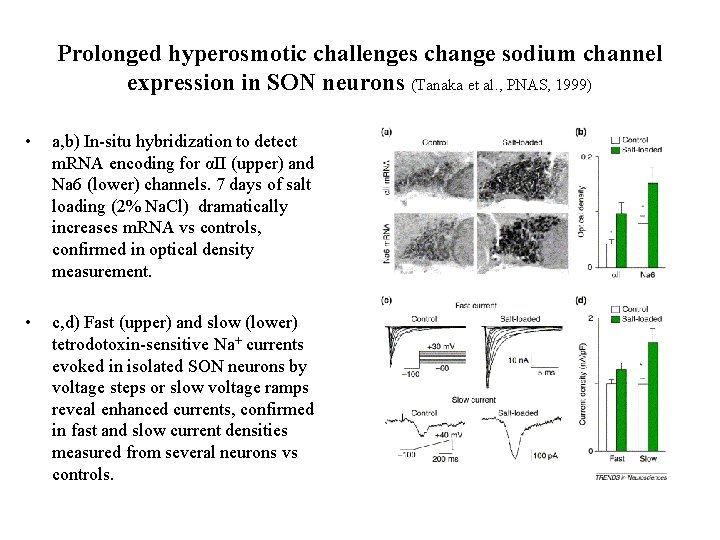
Prolonged hyperosmotic challenges change sodium channel expression in SON neurons (Tanaka et al. , PNAS, 1999) • a, b) In-situ hybridization to detect m. RNA encoding for αII (upper) and Na 6 (lower) channels. 7 days of salt loading (2% Na. Cl) dramatically increases m. RNA vs controls, confirmed in optical density measurement. • c, d) Fast (upper) and slow (lower) tetrodotoxin-sensitive Na+ currents evoked in isolated SON neurons by voltage steps or slow voltage ramps reveal enhanced currents, confirmed in fast and slow current densities measured from several neurons vs controls.
 Adh role
Adh role Antidiuretic hormone function
Antidiuretic hormone function Hyponatremia approach
Hyponatremia approach Erythropin
Erythropin Types of hormone secretion
Types of hormone secretion Hormone secretion
Hormone secretion Thyroid hormone secretion
Thyroid hormone secretion Vasopressin mechanism
Vasopressin mechanism Pancreas
Pancreas Indication of lidocaine
Indication of lidocaine Anti diuretics drugs name
Anti diuretics drugs name Classification of diuretics
Classification of diuretics Entomology is the study of _______.
Entomology is the study of _______. Adh function
Adh function Adh
Adh Iponatriemia
Iponatriemia Adh feedback
Adh feedback What produces adh
What produces adh Dibujo sistema endocrino
Dibujo sistema endocrino Adh vs aldosterone
Adh vs aldosterone Adh released from
Adh released from Nephron diagram labeled
Nephron diagram labeled What does pmi stand for forensic entomology
What does pmi stand for forensic entomology Iponatremie
Iponatremie Regelkring glucose
Regelkring glucose Adh released from
Adh released from Adh function
Adh function How to calculate adh forensics
How to calculate adh forensics Adenohypophysis
Adenohypophysis Difference between adh and aldosterone
Difference between adh and aldosterone Whats adh
Whats adh Capillary
Capillary Sa'd ibn mu'adh
Sa'd ibn mu'adh