Chapter 1 Crystallography 11102020 1 1 Outline 1

















- Slides: 62

Chapter 1 Crystallography 11/10/2020 1 1

Outline 1. Introduction to bonding in solids 2. Types of bonding 3. Classification of solids 4. Basic definitions 5. Crystal Systems and Bravais Lattice 6. Miller Indices and Problems 7. XRD Techniques 11/10/2020 2 2

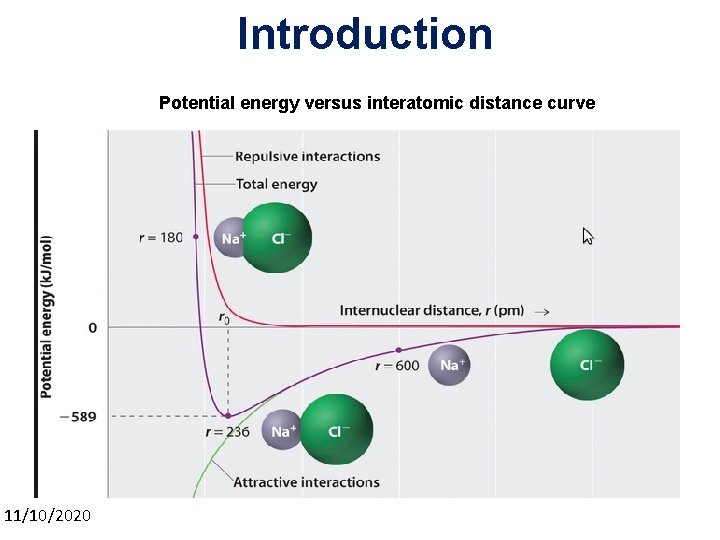
Introduction Potential energy versus interatomic distance curve 11/10/2020 3

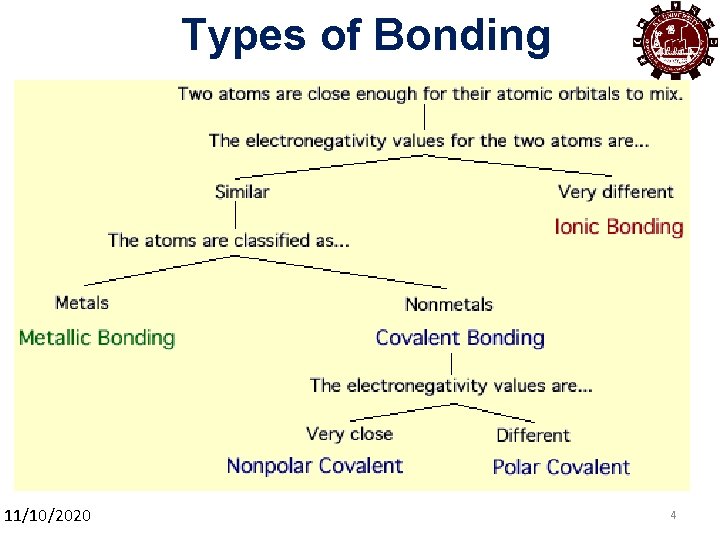
Types of Bonding 11/10/2020 4 4

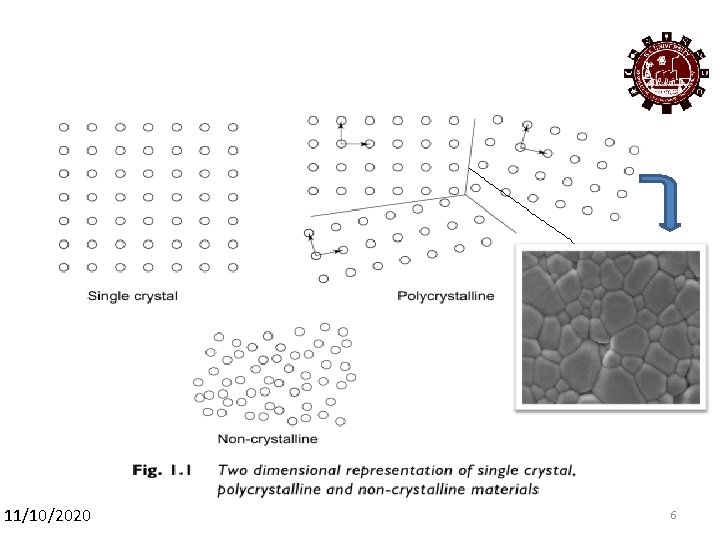
11/10/2020 5

11/10/2020 6 6

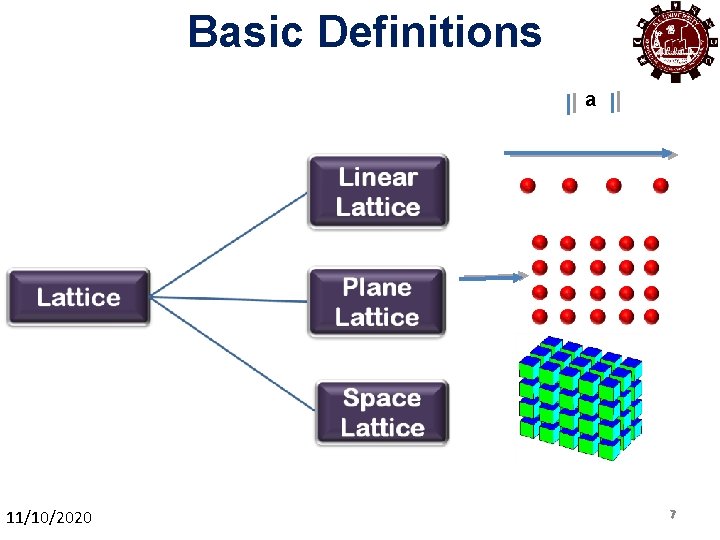
Basic Definitions a 11/10/2020 77

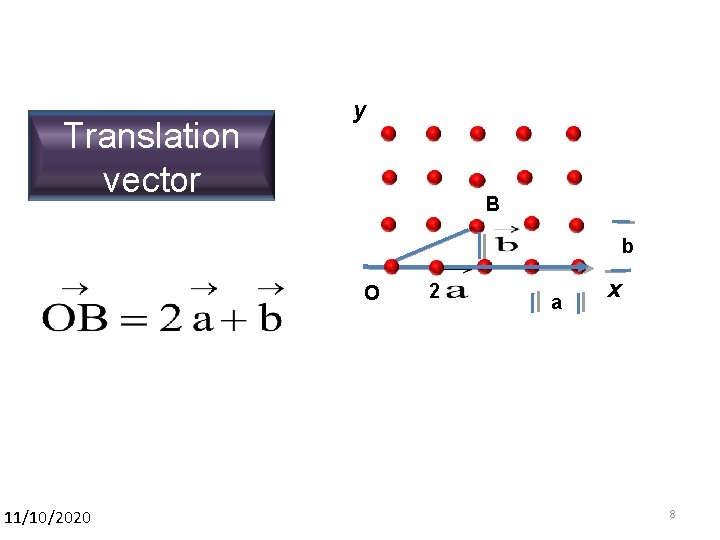
Translation vector y B b O 11/10/2020 2 a x 8

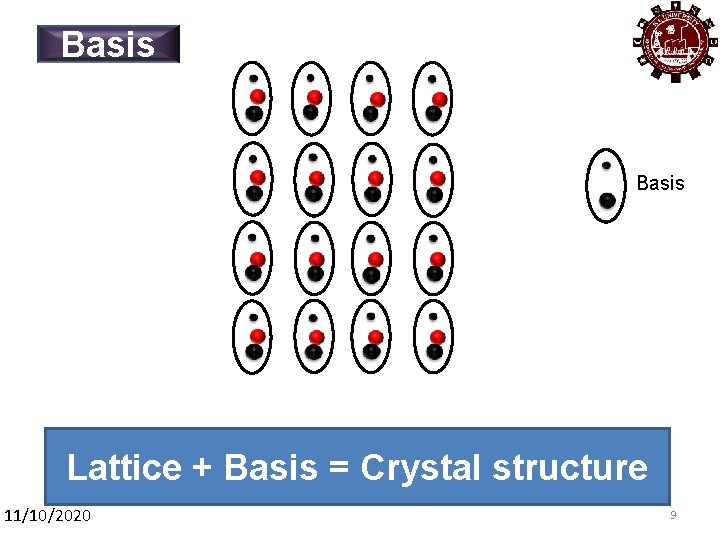
Basis Lattice + Basis = Crystal structure 11/10/2020 9 9

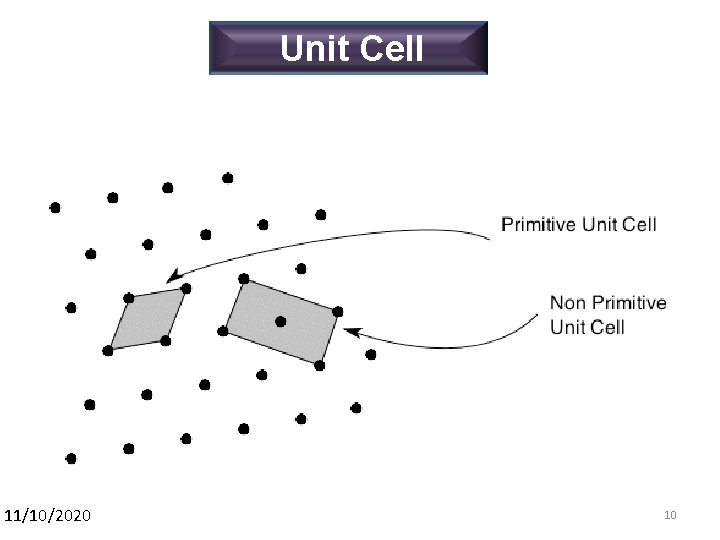
Unit Cell 11/10/2020 10 10

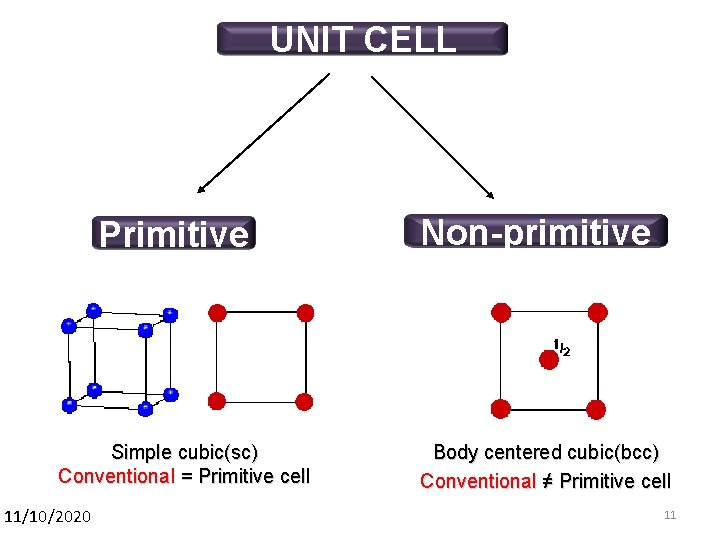
UNIT CELL Primitive Simple cubic(sc) Conventional = Primitive cell 11/10/2020 Non-primitive Body centered cubic(bcc) Conventional ≠ Primitive cell 11 11

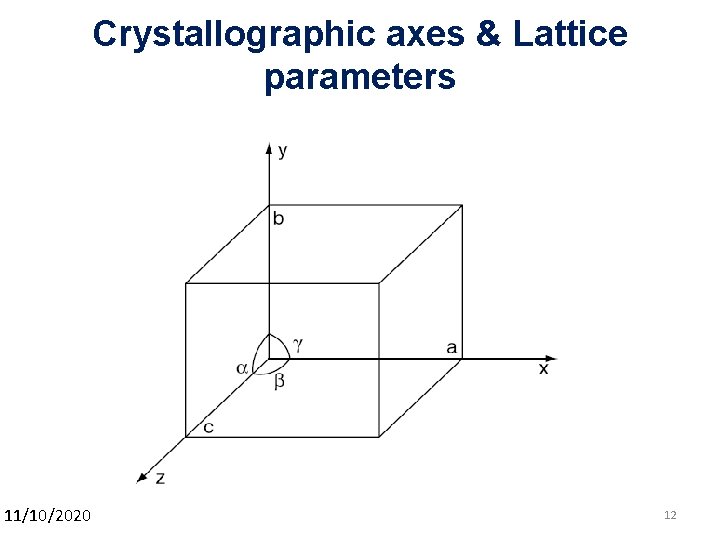
Crystallographic axes & Lattice parameters 11/10/2020 12 12

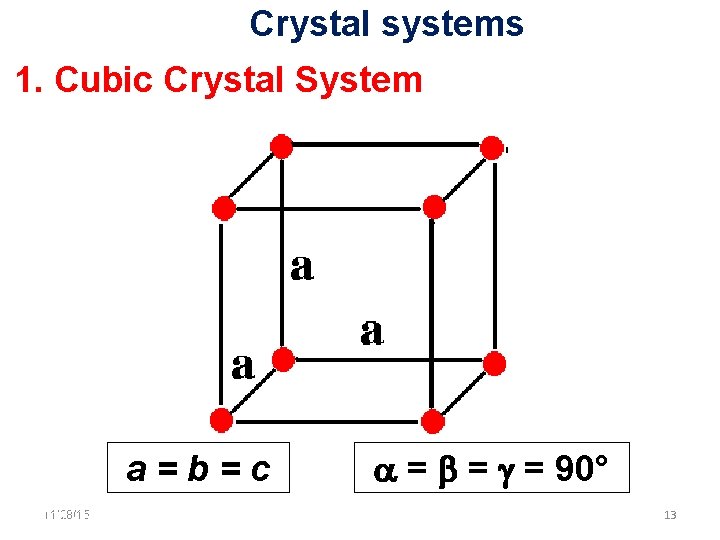
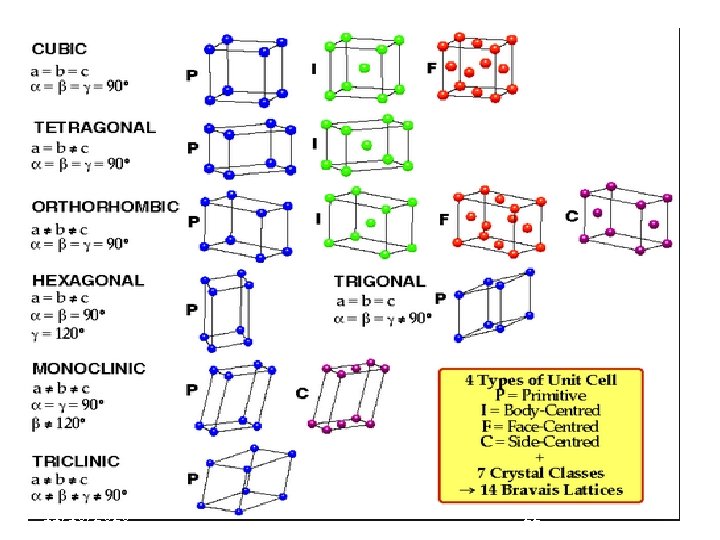
Crystal systems 1. Cubic Crystal System a=b=c 11/28/15 11/10/2020 = = = 90° 13 13

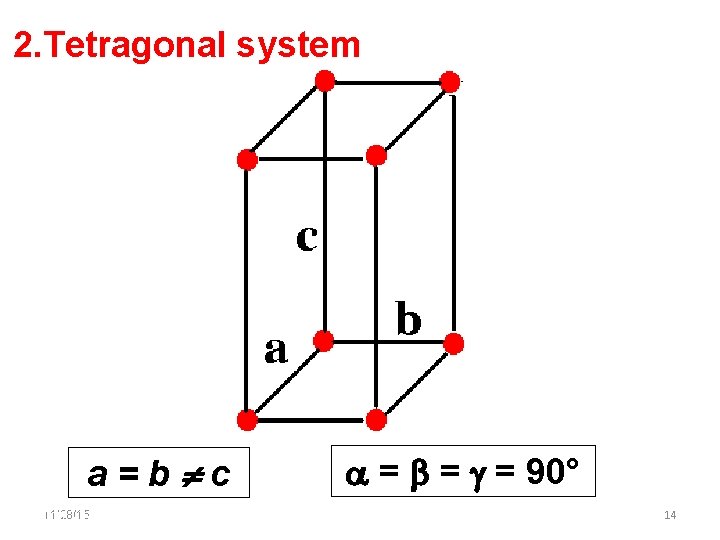
2. Tetragonal system a=b c 11/28/15 11/10/2020 = = = 90° 14 14

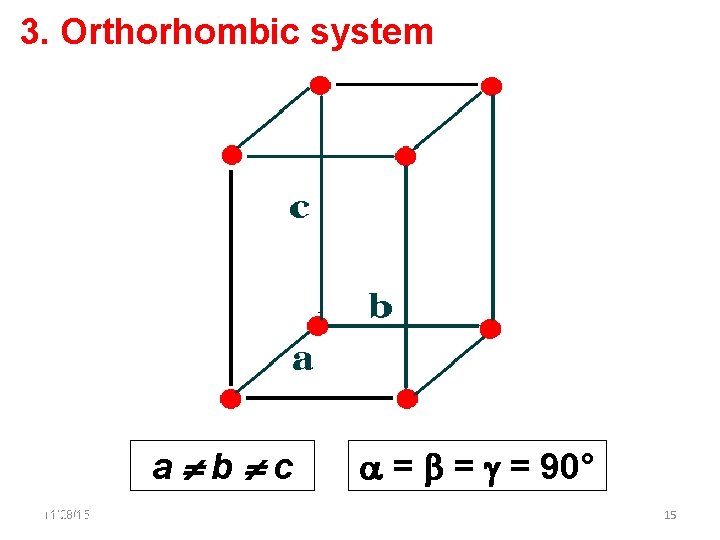
3. Orthorhombic system a b c 11/28/15 11/10/2020 = = = 90° 15 15

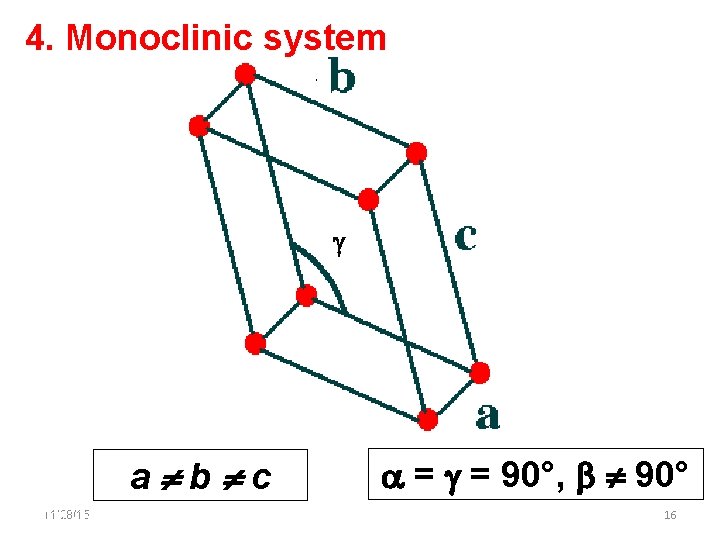
4. Monoclinic system a b c 11/28/15 11/10/2020 = = 90°, 90° 16 16

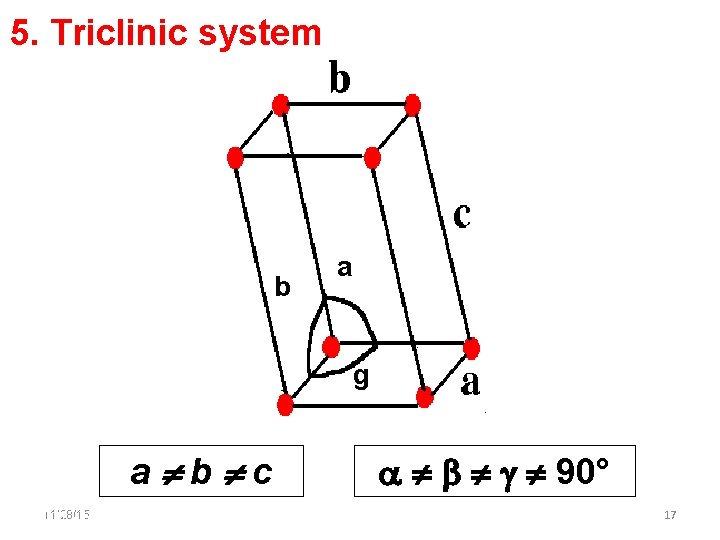
5. Triclinic system b a g a b c 11/28/15 11/10/2020 90° 17 17

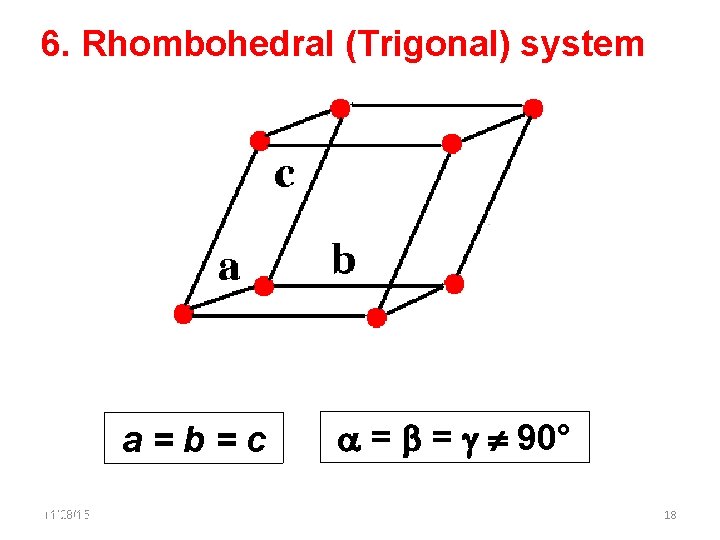
6. Rhombohedral (Trigonal) system a=b=c 11/28/15 11/10/2020 = = 90° 18 18

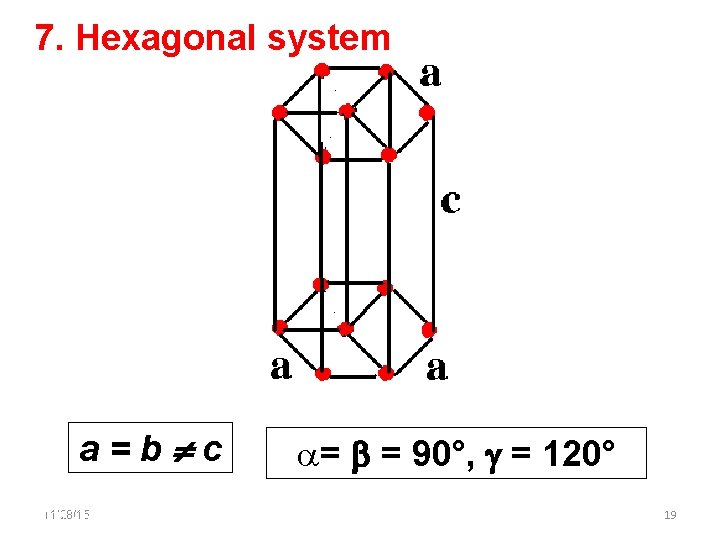
7. Hexagonal system a=b c 11/28/15 11/10/2020 = = 90°, = 120° 19 19

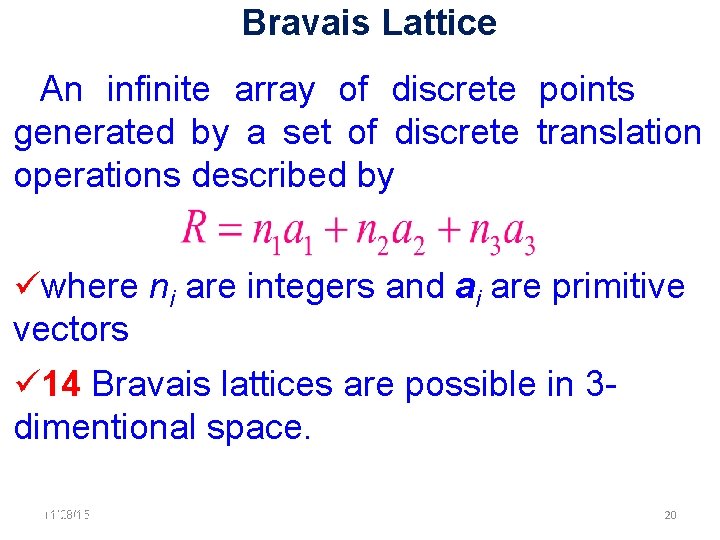
Bravais Lattice An infinite array of discrete points generated by a set of discrete translation operations described by where ni are integers and ai are primitive vectors 14 Bravais lattices are possible in 3 dimentional space. 11/28/15 11/10/2020 20 20

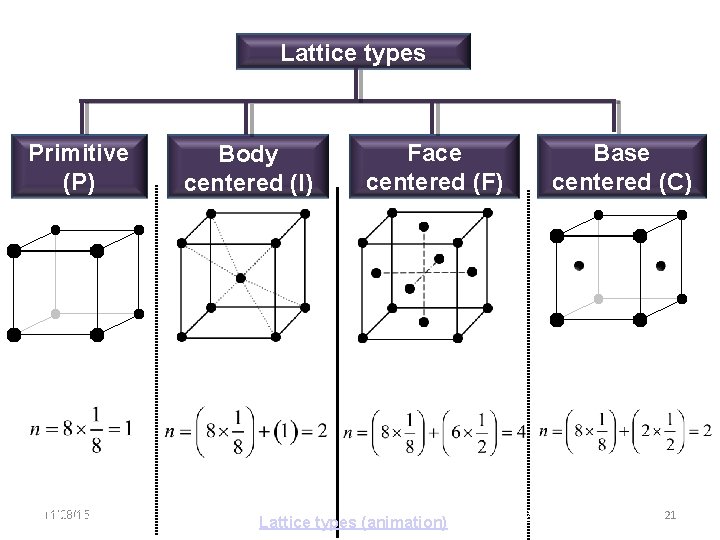
Lattice types Primitive (P) 11/28/15 11/10/2020 Body centered (I) Base centered (C) Face centered (F) Lattice types (animation) 21 21

11/10/2020 22

11/28/15 11/10/2020 23 23

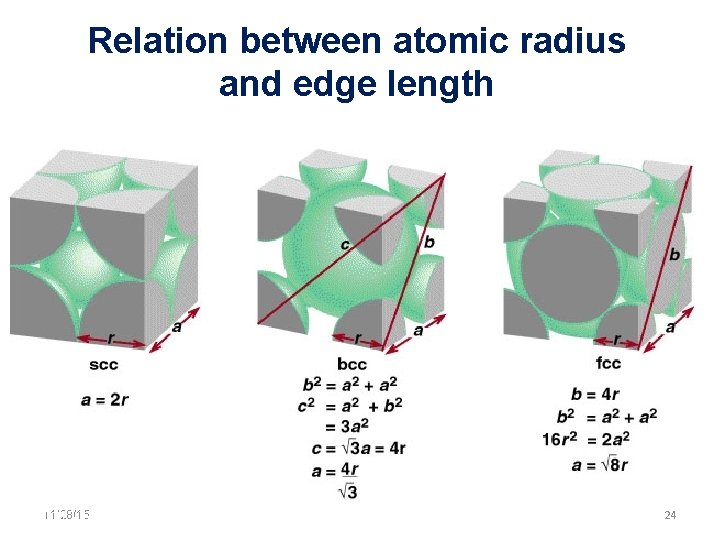
Relation between atomic radius and edge length 11/28/15 11/10/2020 24 24

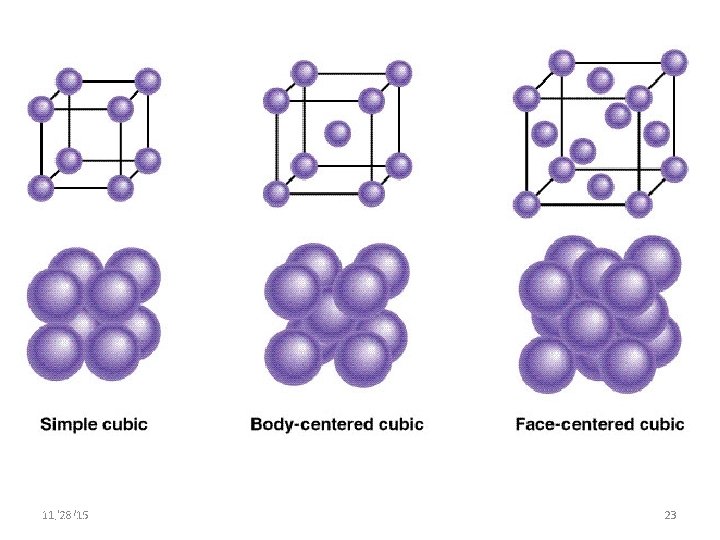
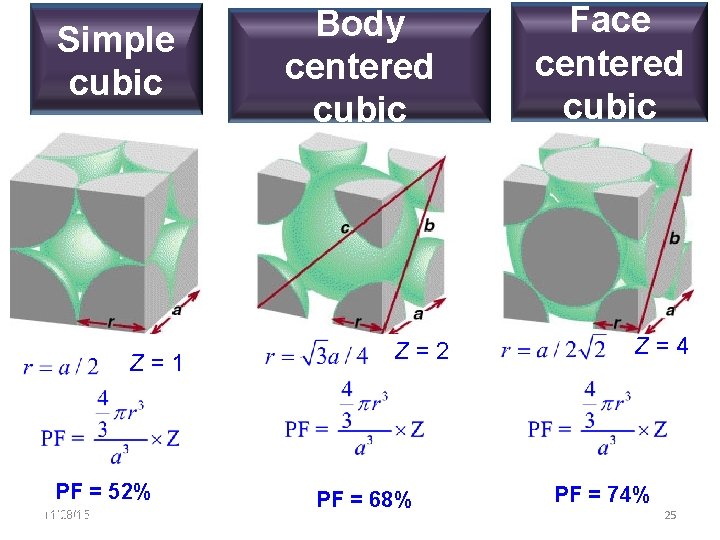
Simple cubic Z=1 PF = 52% 11/28/15 11/10/2020 Body centered cubic Face centered cubic Z=4 Z=2 PF = 68% 25 PF = 74% 25

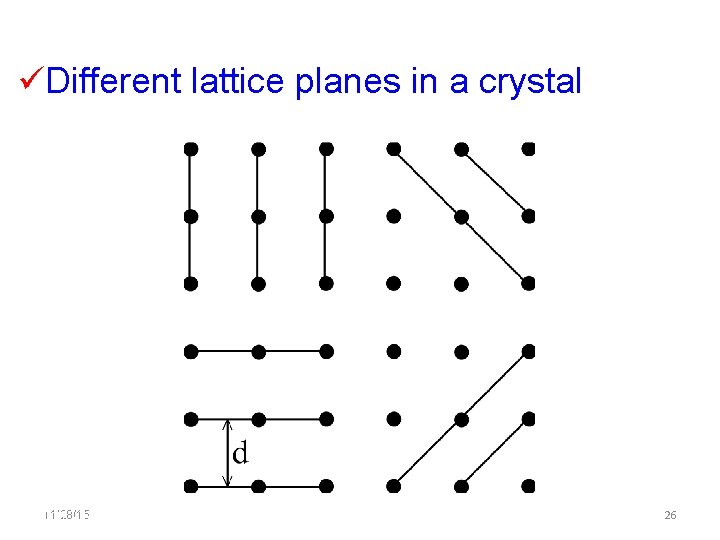
Different lattice planes in a crystal 11/28/15 11/10/2020 26 26

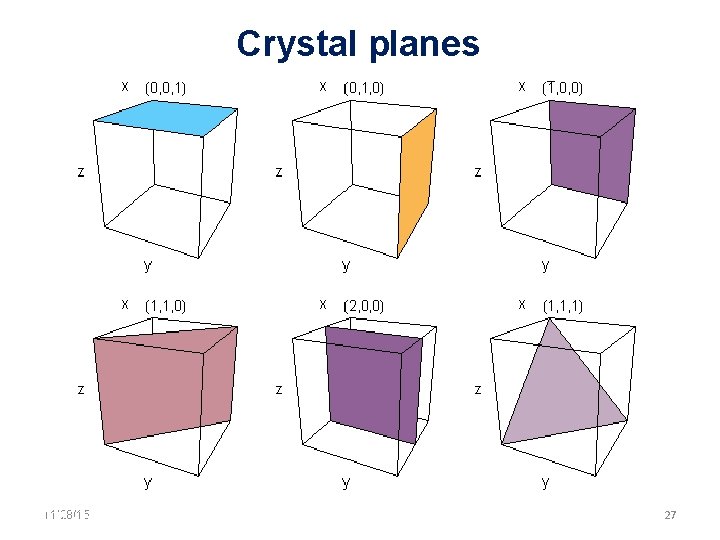
Crystal planes 11/28/15 11/10/2020 27 27

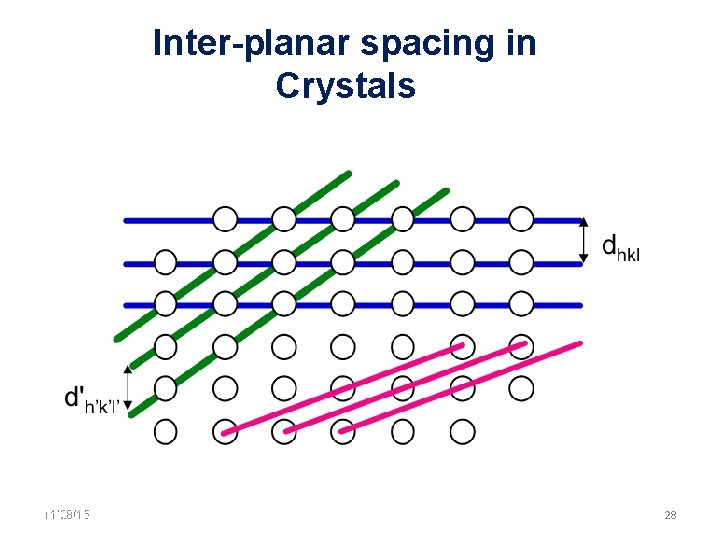
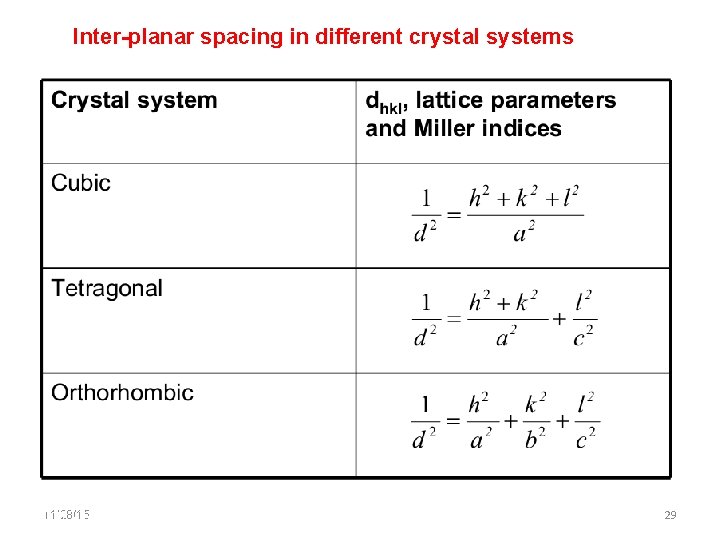
Inter-planar spacing in Crystals 11/28/15 11/10/2020 28 28

Inter-planar spacing in different crystal systems 11/28/15 11/10/2020 29 29

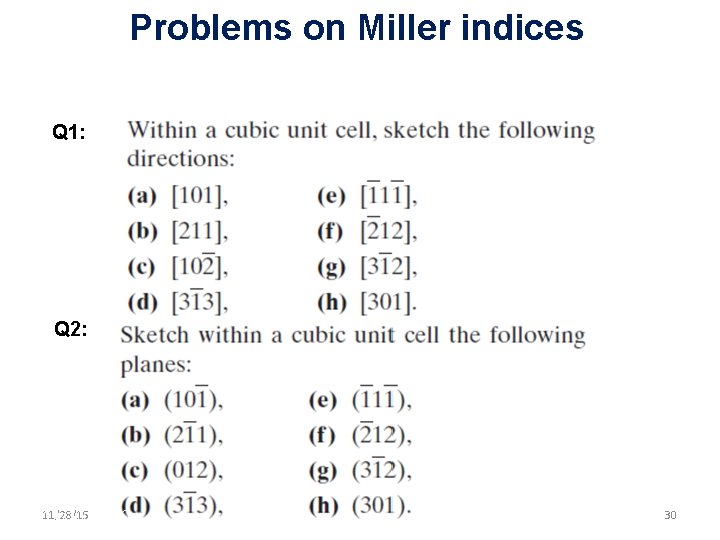
Problems on Miller indices Q 1: Q 2: 11/28/15 11/10/2020 30 30

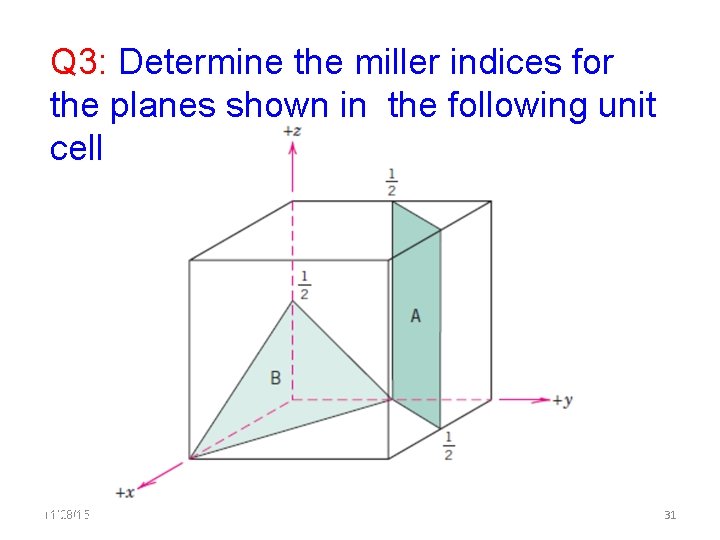
Q 3: Determine the miller indices for the planes shown in the following unit cell 11/28/15 11/10/2020 31 31

Q 4: What are Miller Indices? Draw (111) and (110) planes in a cubic lattice. Q 5: Sketch the following planes of a cubic unit cell (001), (120), (211) Q 6: Obtain the Miller indices of a plane which intercepts at a, b/2 and 3 c in simple cubic unit cell. Draw a neat diagram 11/10/2020 32 11/28/15 32

Problems on inter-planar spacing 1. Explain how the X-ray diffraction can be employed to determine the crystal structure. Give the ratio of inter-planar distances of (100), (110) and (111) planes for a simple cubic structure. 11/28/15 11/10/2020 33 33

2. The distance between (110) planes in a body centered cubic structure is 0. 203 nm. What is the size of the unit cell? What is the radius of the atom? 11/10/2020 34

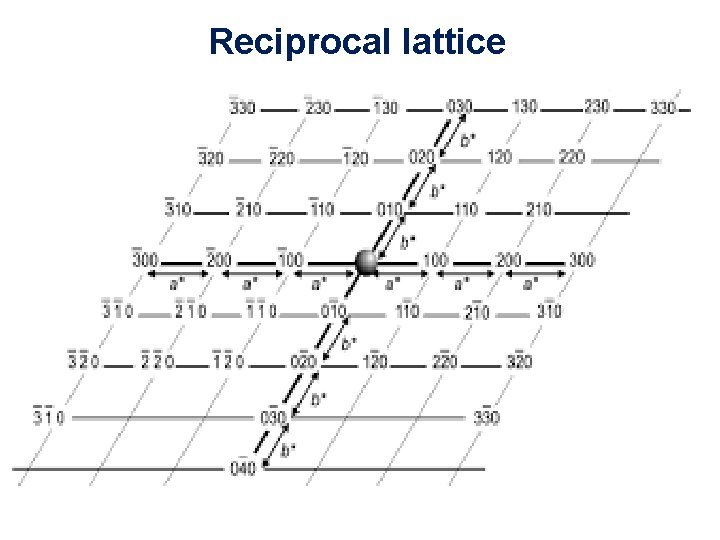
Reciprocal lattice 11/10/2020 35

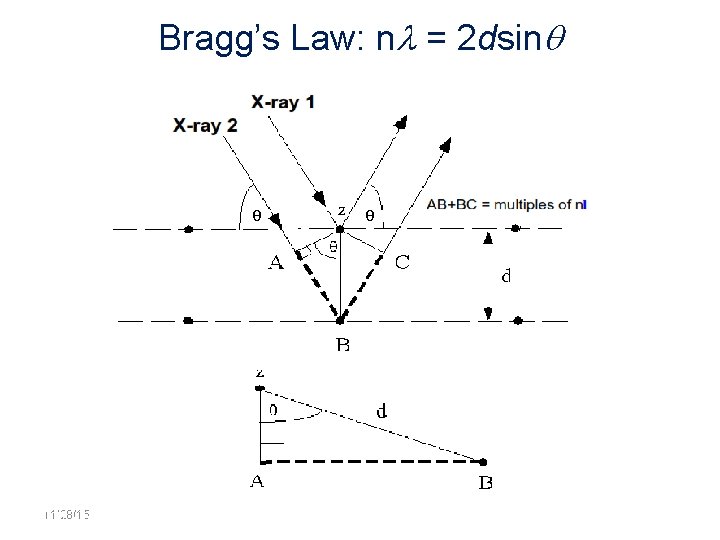
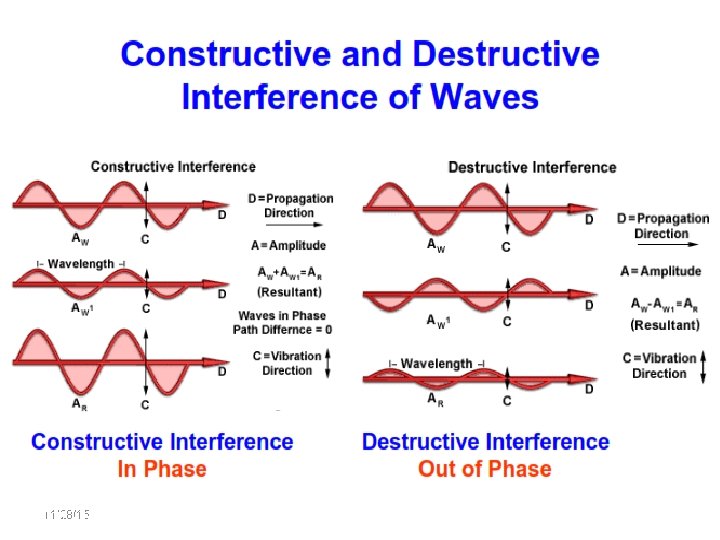
Bragg’s Law: n = 2 dsin 11/28/15 11/10/2020 36

11/28/15 11/10/2020 37

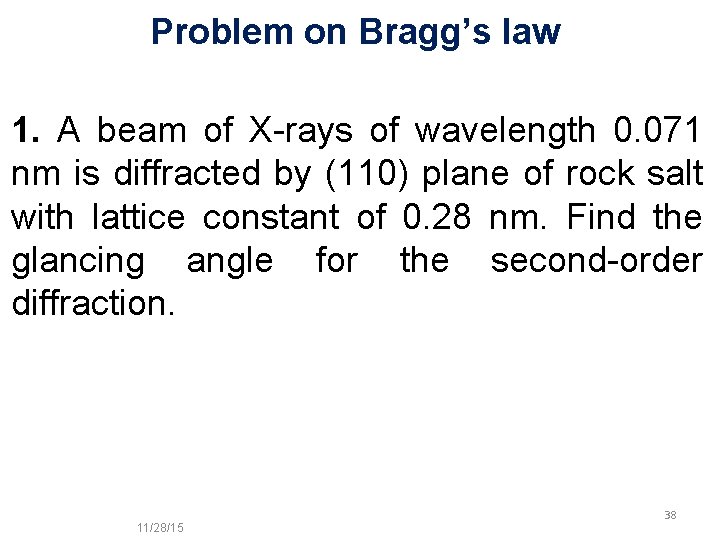
Problem on Bragg’s law 1. A beam of X-rays of wavelength 0. 071 nm is diffracted by (110) plane of rock salt with lattice constant of 0. 28 nm. Find the glancing angle for the second-order diffraction. 11/10/2020 11/28/15 38 38

Problem on Bragg’s law 2. A beam of X-rays is incident on a Na. Cl crystal with lattice plane spacing 0. 282 nm. Calculate the wavelength of X-rays if the first-order Bragg reflection takes place at a glancing angle of 8 ° 35′. Also calculate the maximum order of diffraction possible. 11/10/2020 39

Problem on Bragg’s law 3. Monochromatic X-rays of λ = 1. 5 A. U are incident on a crystal face having an interplanar spacing of 1. 6 A. U. Find the highest order for which Bragg’s reflection maximum can be seen. 11/10/2020 40

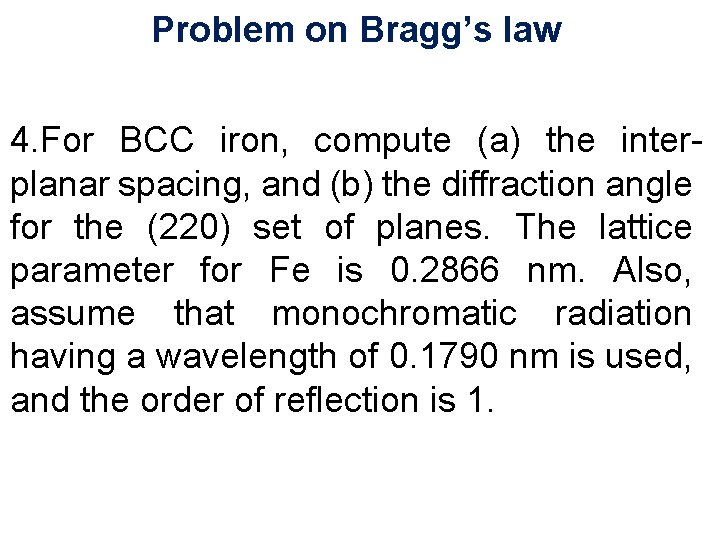
Problem on Bragg’s law 4. For BCC iron, compute (a) the interplanar spacing, and (b) the diffraction angle for the (220) set of planes. The lattice parameter for Fe is 0. 2866 nm. Also, assume that monochromatic radiation having a wavelength of 0. 1790 nm is used, and the order of reflection is 1. 11/10/2020 41

Problem on Bragg’s law 5. The metal niobium has a BCC crystal structure. If the angle of diffraction for the (211) set of planes occurs at 75. 99 o (first order reflection) when monochromatic Xradiation having a wavelength 0. 1659 nm is used. Compute (a) the inter-planar spacing for this set of planes and (b) the atomic radius for the niobium atom. 11/10/2020 42

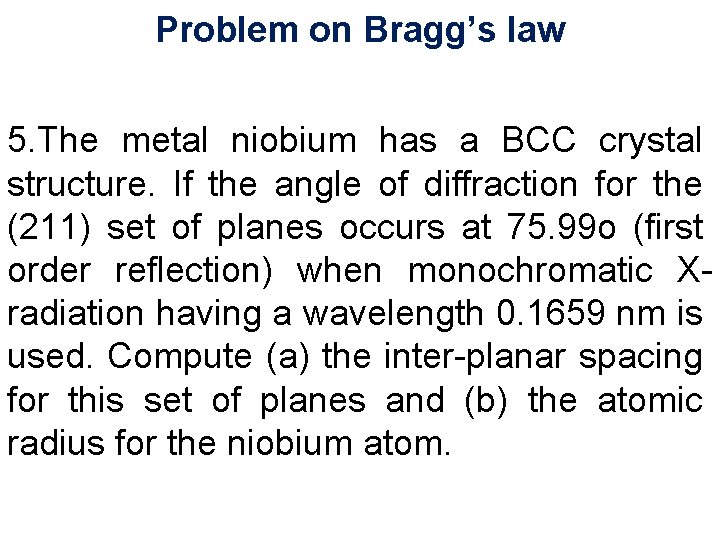
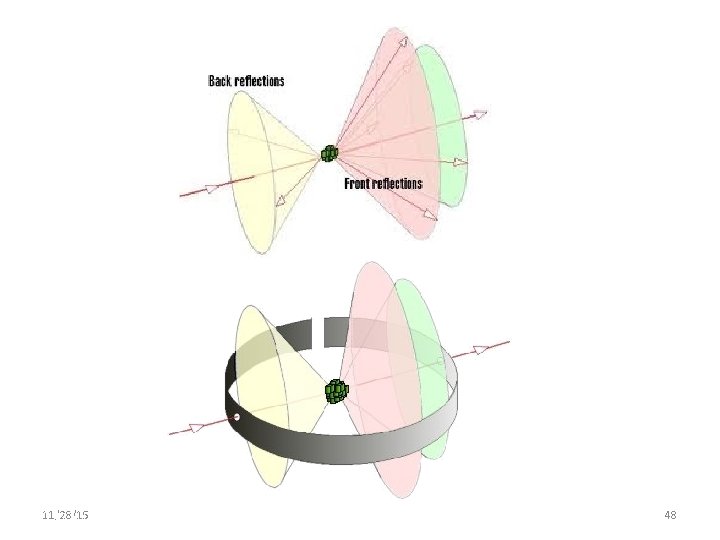
Laue Method Single Collimator crystal D F 2 r 1 X-rays B S D Transmission Method 11/28/15 11/10/2020 43 43

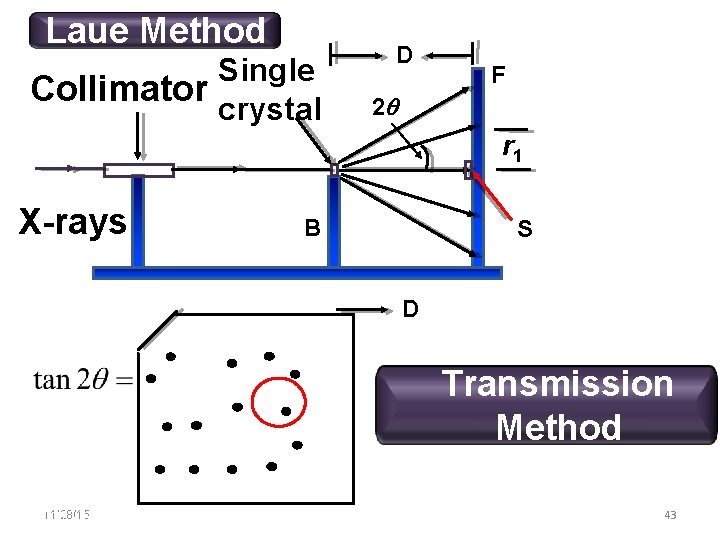
Back-reflection method Collimator Single crystal (180 -2 ) F r 2 B S D 11/10/2020 44

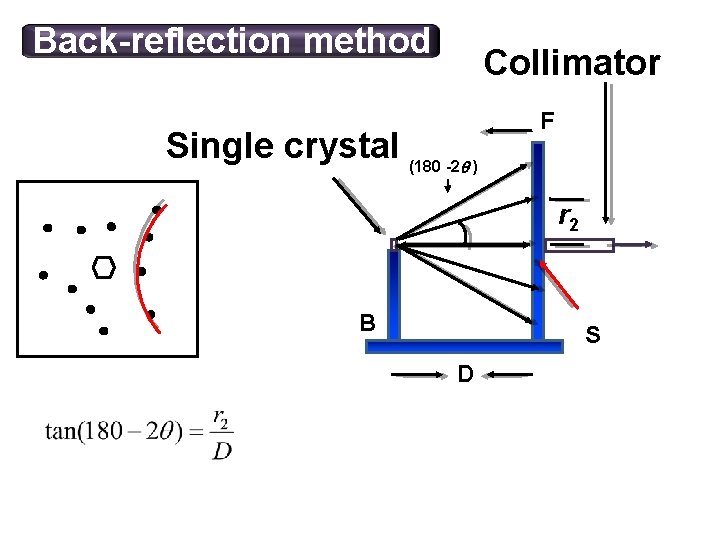
Transmission method 11/28/15 11/10/2020 Back-reflection method 45 45

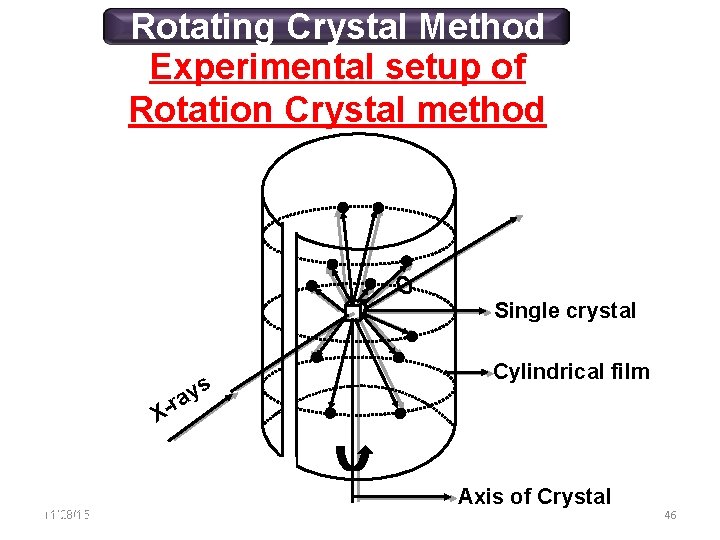
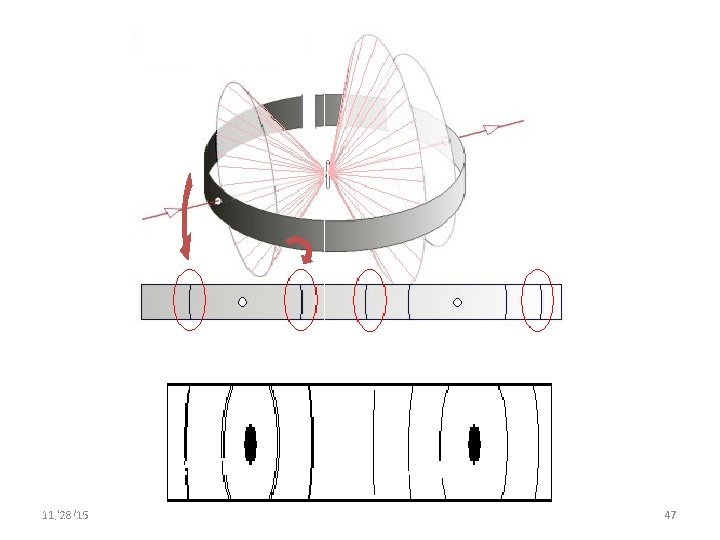
Rotating Crystal Method Experimental setup of Rotation Crystal method Single crystal s y -ra Cylindrical film X 11/28/15 11/10/2020 Axis of Crystal 46 46

11/28/15 11/10/2020 47 47

11/28/15 11/10/2020 48 48

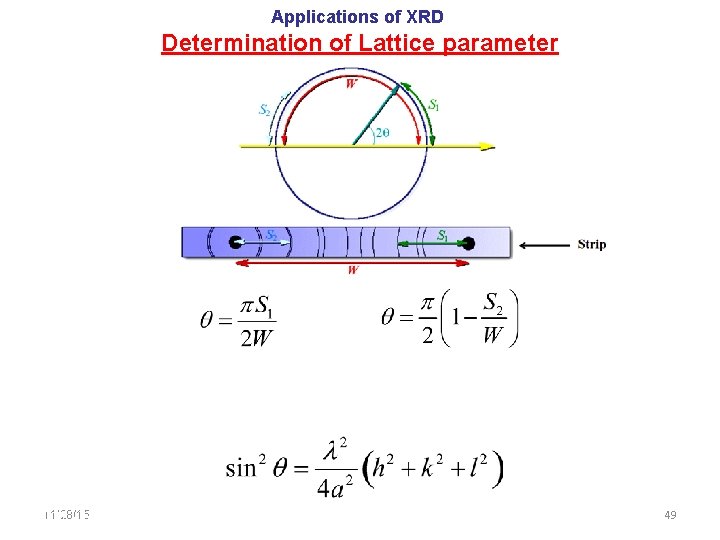
Applications of XRD Determination of Lattice parameter 11/28/15 11/10/2020 49 49

X-Ray Diffraction technique is used to Distinguishing between crystalline & amorphous materials. Determination of the crystalline materials. structure of Determination of electron distribution within the atoms, & throughout the unit cell. 11/28/15 11/10/2020 50 Confidential 50

Determination of the orientation of single crystals. Determination of the polygrained materials. texture of Measurement of strain and small grain size…. . etc. 11/10/2020 51

Advantages & Disadvantages of X-Ray Diffraction Advantages XRD is a nondestructive technique. X-Rays are the least expensive, the most convenient & the most widely used method to determine crystal structures. 11/28/15 11/10/2020 52 Confidential 52

X-Rays are not absorbed very much by air, so the sample need not be in an evacuated chamber. Disadvantages X-Rays do not interact very strongly with lighter elements. 11/10/2020 53

Problems 1. Chromium has BCC structure. Its atomic radius is 0. 1249 nm. Calculate the free volume / unit cell. 2. Lithium crystallizes in BCC structure. Calculate the lattice constant, given that the atomic weight and density for lithium are 6. 94 and 530 kg/m 3 respectively. 11/28/15 11/10/2020 54 Confidential 54

3. Iron crystallizes in BCC structure. Calculate the lattice constant, given that the atomic weight and density of iron are 55. 85 and 7860 kg/m 3 respectively. 4. If the edge of the unit cell of a cube in the diamond structure is 0. 356 nm, calculate the number of atoms/m 3. 11/10/2020 55

5. A metal in BCC structure has a lattice constant 3. 5 Ao. Calculate the number of atoms per sq. mm area in the (200) plane. 6. Germanium crystallizes in diamond (from) structures with 8 atoms per unit cell. If the lattice constant is 5. 62 Ao, calculate its density. 11/10/2020 56

7. A beam of X-rays of wavelength 0. 071 nm is diffracted by (100) plane of rock salt with lattice constant of 0. 28 nm. Find the glancing angle for the second – order diffraction. 11/28/15 11/10/2020 57 Confidential 57

8. A beam of X-rays is incident on a Na. Cl crystal with lattice plane spacing 0. 282 nm. Calculate the wavelength of X-rays if the first-order Bragg reflection takes place at a glancing angle of 8 o 35’. Also calculate the maximum order of diffraction possible. 9. The fraction of vacant sites in a metal is 1 X 10 -10 at 500 o. C. What will be the fraction of vacancy sites at 1000 o C? 11/28/15 11/10/2020 58 Confidential 58

10. Calculate the ratios of d 100 : d 111 for a simple cubic structure 11. The Bragg’s angle in the first order for (220) reflection from nickel (FCC) is 38. 2 o. When X-rays of wavelength 1. 54 Ao are employed in a diffraction experiment. Determine the lattice parameter of nickel. 11/10/2020 59

12. Monochromatic X-rays of = 1. 5 A. U are incident on a crystal face having an inter-planar spacing of 1. 6 A. U. Find the highest order for which Bragg’s reflection maximum can be seen. 13. Copper has FCC structure with lattice constant 0. 36 nm. Calculate the inter-planar spacing for (111) and (321) planes. 11/10/2020 60

14. The distance between (100) planes in a BCC structure is 0. 203 nm. What is the size of the unit cell? What is the radius of the atom? 15. Monochromatic X-rays of = 1. 5 A. U are incident on a crystal face having an inter-planar spacing of 1. 6 A. U. Find the highest order for which Bragg’s reflection maximum can be seen. 11/10/2020 61

16. The first order diffraction occurs when a X-ray beam of wavelength 0. 675 Ao incident at a glancing angle 5 o 25’ on a crystal. What is the glancing angle for third -order diffraction to occur? 17. The Bragg’s angle in the first order for (220) reflection from nickel ( FCC) is 38. 2 o. When X-rays of wavelength 1. 54 Ao are employed in a diffraction experiment. Determine the lattice parameter of nickel. 11/10/2020 62
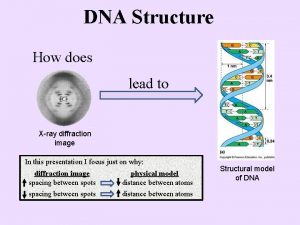
 Dna x-ray crystallography
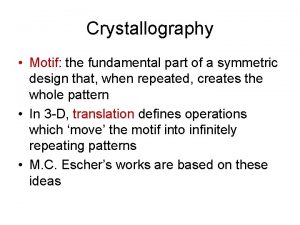
Dna x-ray crystallography Motif crystal
Motif crystal Site:slidetodoc.com
Site:slidetodoc.com Stereographic projection crystallography
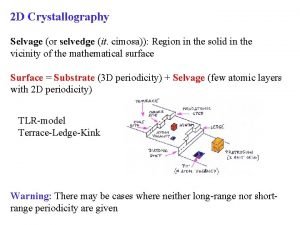
Stereographic projection crystallography Crystallography types
Crystallography types Crystalline solid
Crystalline solid Crystallography engineering physics
Crystallography engineering physics Jana crystallography
Jana crystallography Crystallography
Crystallography Crystallography
Crystallography Crystallography information file
Crystallography information file Xds crystallography
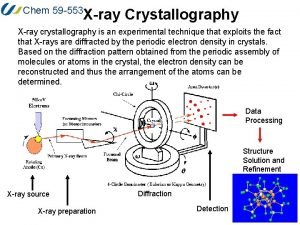
Xds crystallography Xrd
Xrd Cod crystallography
Cod crystallography Sandwich quotes examples
Sandwich quotes examples Discourage criminal acts by threatening punishment
Discourage criminal acts by threatening punishment Romans outline by chapter
Romans outline by chapter Drafting a research proposal
Drafting a research proposal Give me liberty chapter 27 outline
Give me liberty chapter 27 outline Secondary data
Secondary data Chapter 38 a world without borders outline
Chapter 38 a world without borders outline Vbscript
Vbscript Hunger games chapter 6 questions and answers
Hunger games chapter 6 questions and answers Chapter 31 societies at crossroads
Chapter 31 societies at crossroads General hero
General hero Observation research method
Observation research method Chapter 1 outline
Chapter 1 outline Chapter 1 outline
Chapter 1 outline Chapter 30 section 5 outline map the vietnam war
Chapter 30 section 5 outline map the vietnam war Chapter 2 outline
Chapter 2 outline The book of acts outline
The book of acts outline Government spending multiplier
Government spending multiplier 24 chapter outline
24 chapter outline Forty niners apush
Forty niners apush Rhetorical precis template
Rhetorical precis template Leq complexity point
Leq complexity point Block method outline
Block method outline Social psychology outline
Social psychology outline Lesson outline lesson 3 describing circuits answers
Lesson outline lesson 3 describing circuits answers Brainpop protists
Brainpop protists Outline of the book of numbers
Outline of the book of numbers The physical outline of a display
The physical outline of a display Lesson outline lesson 3 mountain building answers
Lesson outline lesson 3 mountain building answers Lesson outline lesson 2 aquatic ecosystems answer key
Lesson outline lesson 2 aquatic ecosystems answer key Enduring issue essay outline
Enduring issue essay outline Us bank api
Us bank api Army aar questions
Army aar questions Psalm 61 sermon outline
Psalm 61 sermon outline Psalm 46 sermon outline
Psalm 46 sermon outline Psalm 40 sermon outline
Psalm 40 sermon outline Pathology outline
Pathology outline Example of thesis statement
Example of thesis statement Jesus vs ganesha
Jesus vs ganesha Toulmin paragraph example
Toulmin paragraph example Synthesis essay
Synthesis essay Where is colossae in the bible
Where is colossae in the bible Crisis cycle mandt
Crisis cycle mandt Presentation outline slide
Presentation outline slide Case brief outline
Case brief outline 1 corinthians outline
1 corinthians outline 2 corinthians outline
2 corinthians outline Outline of 1 corinthians
Outline of 1 corinthians Cognitive model psychology
Cognitive model psychology