Lecture 20 XRay Diffraction XRD o Theory and

- Slides: 14

Lecture 20 X-Ray Diffraction (XRD) o Theory and Analytical Technique

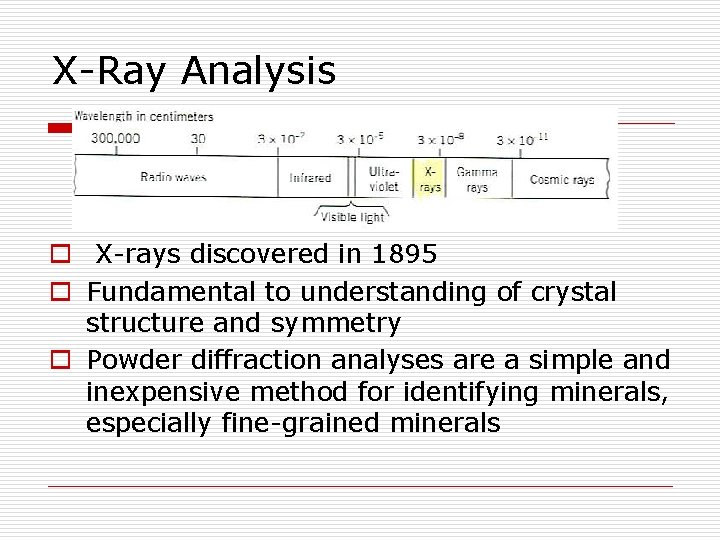
X-Ray Analysis o X-rays discovered in 1895 o Fundamental to understanding of crystal structure and symmetry o Powder diffraction analyses are a simple and inexpensive method for identifying minerals, especially fine-grained minerals

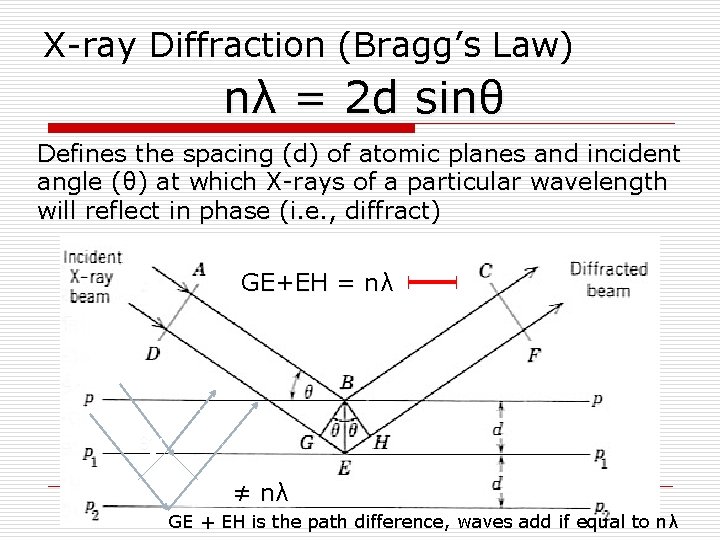
X-ray Crystallography X-ray wavelengths are on the same order of magnitude as atomic spacings. Crystals thus make excellent diffraction gratings Can use the geometry of the x-ray spots to determine geometry of grating (i. e. the crystal) nλ = 2 d sin θ

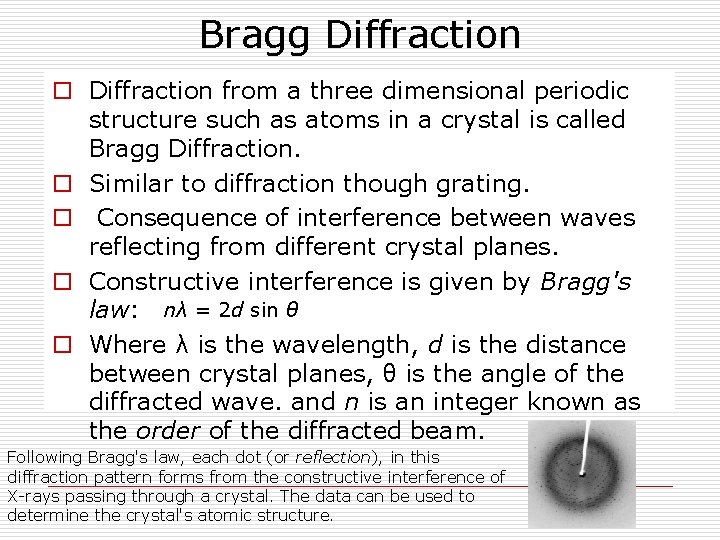
Bragg Diffraction o Diffraction from a three dimensional periodic structure such as atoms in a crystal is called Bragg Diffraction. o Similar to diffraction though grating. o Consequence of interference between waves reflecting from different crystal planes. o Constructive interference is given by Bragg's law: nλ = 2 d sin θ o Where λ is the wavelength, d is the distance between crystal planes, θ is the angle of the diffracted wave. and n is an integer known as the order of the diffracted beam. Following Bragg's law, each dot (or reflection), in this diffraction pattern forms from the constructive interference of X-rays passing through a crystal. The data can be used to determine the crystal's atomic structure.

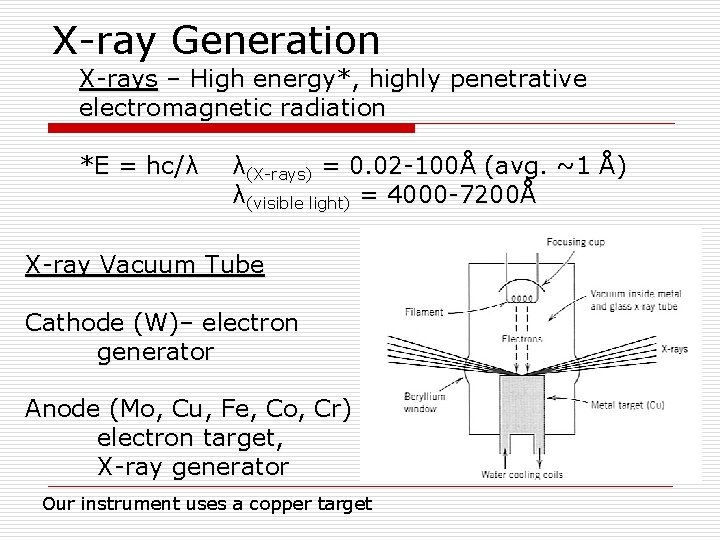
X-ray Generation X-rays – High energy*, highly penetrative electromagnetic radiation *E = hc/λ λ(X-rays) = 0. 02 -100Å (avg. ~1 Å) λ(visible light) = 4000 -7200Å X-ray Vacuum Tube Cathode (W)– electron generator Anode (Mo, Cu, Fe, Co, Cr) – electron target, X-ray generator Our instrument uses a copper target

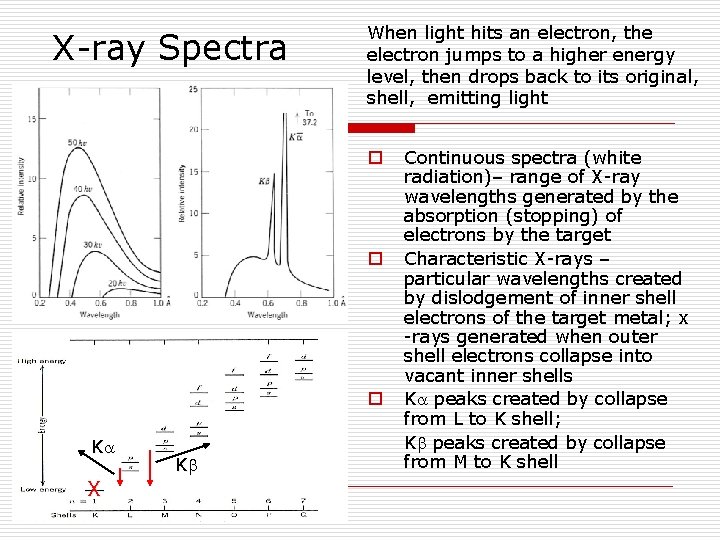
X-ray Spectra When light hits an electron, the electron jumps to a higher energy level, then drops back to its original, shell, emitting light o o o K X K Continuous spectra (white radiation)– range of X-ray wavelengths generated by the absorption (stopping) of electrons by the target Characteristic X-rays – particular wavelengths created by dislodgement of inner shell electrons of the target metal; x -rays generated when outer shell electrons collapse into vacant inner shells K peaks created by collapse from L to K shell; K peaks created by collapse from M to K shell

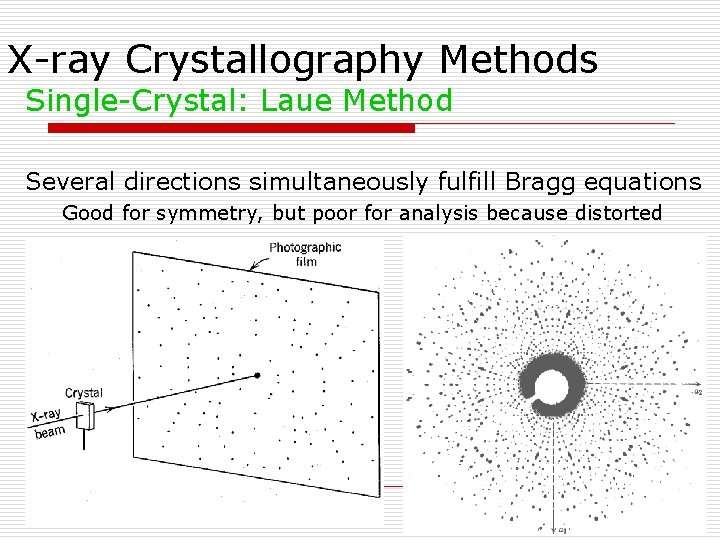
X-ray Crystallography Methods Single-Crystal: Laue Method Several directions simultaneously fulfill Bragg equations Good for symmetry, but poor for analysis because distorted Fig 7. 39 of Klein (2002) Manual of Mineral Science, John Wiley and Sons

X-ray Diffraction (Bragg’s Law) nλ = 2 d sinθ Defines the spacing (d) of atomic planes and incident angle (θ) at which X-rays of a particular wavelength will reflect in phase (i. e. , diffract) GE+EH = nλ θ’ ≠ nλ GE + EH is the path difference, waves add if equal to nλ

X-ray Crystallography Methods: Single-Crystal: Precession Use motors to move crystal & sensor to satisfy Bragg equations for different planes without distortions Fig 7. 40 of Klein (2002) Manual of Mineral Science, John Wiley and Sons

X-ray Crystallography Methods Powder Easiest Infinite orientations at once, so only need to vary q , the angle of the incident beam of x-ray light.

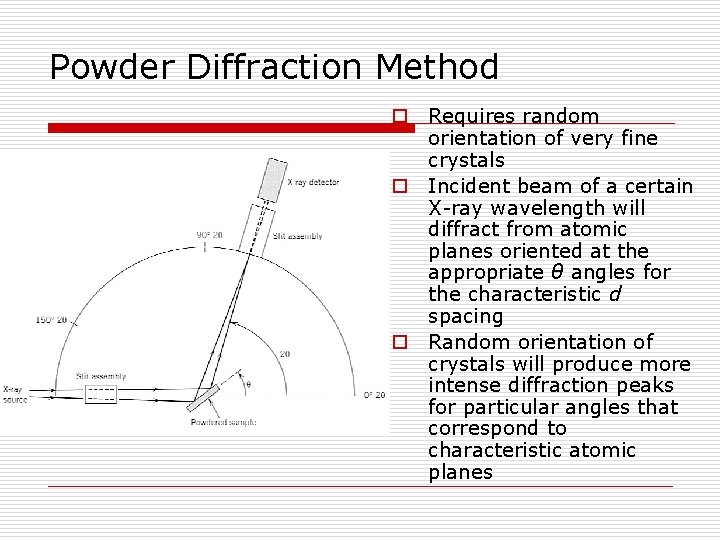
Powder Diffraction Method o Requires random orientation of very fine crystals o Incident beam of a certain X-ray wavelength will diffract from atomic planes oriented at the appropriate θ angles for the characteristic d spacing o Random orientation of crystals will produce more intense diffraction peaks for particular angles that correspond to characteristic atomic planes

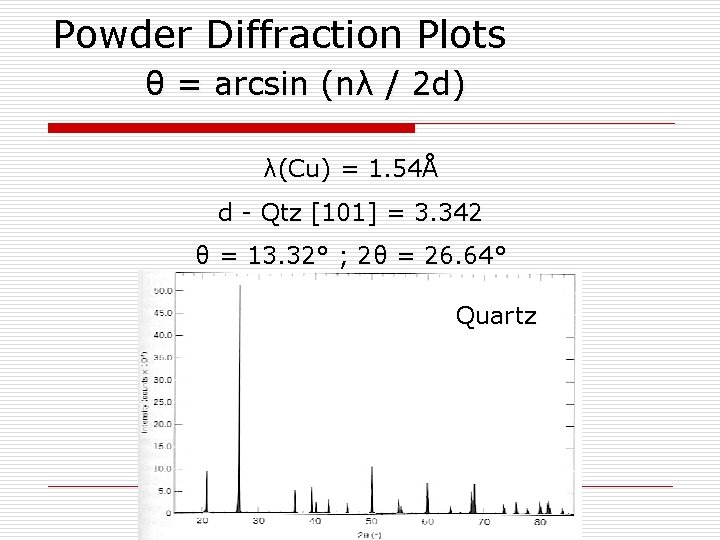
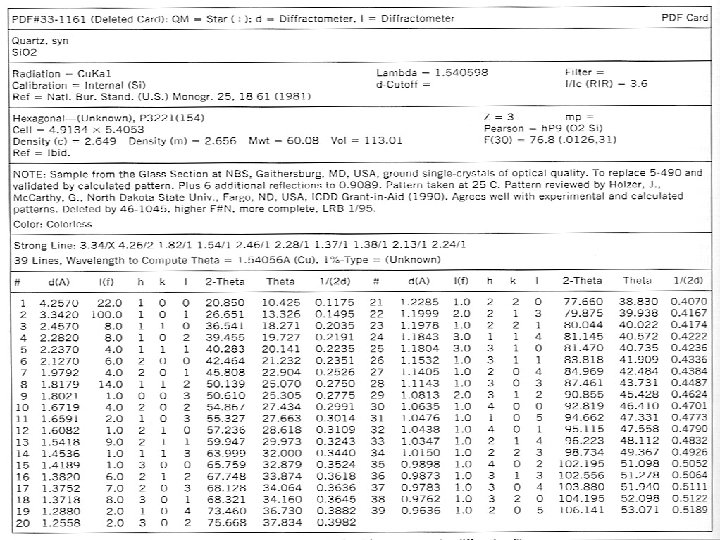
Powder Diffraction Plots θ = arcsin (nλ / 2 d) λ(Cu) = 1. 54Å d - Qtz [101] = 3. 342 θ = 13. 32° ; 2θ = 26. 64° Quartz


Interpreting X-ray data o We will use the data obtained to n identify the mineral n determine the dimensions of the unit cell.