Calculating Energy Changes at Phase Changes The heating





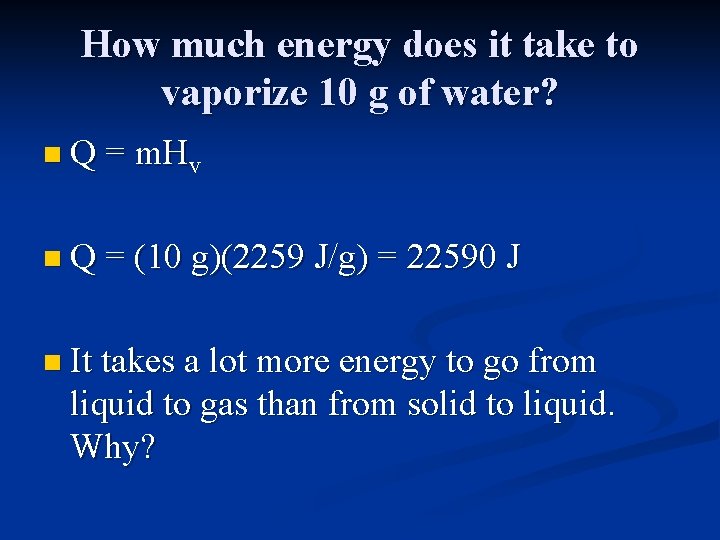




- Slides: 17

Calculating Energy Changes at Phase Changes

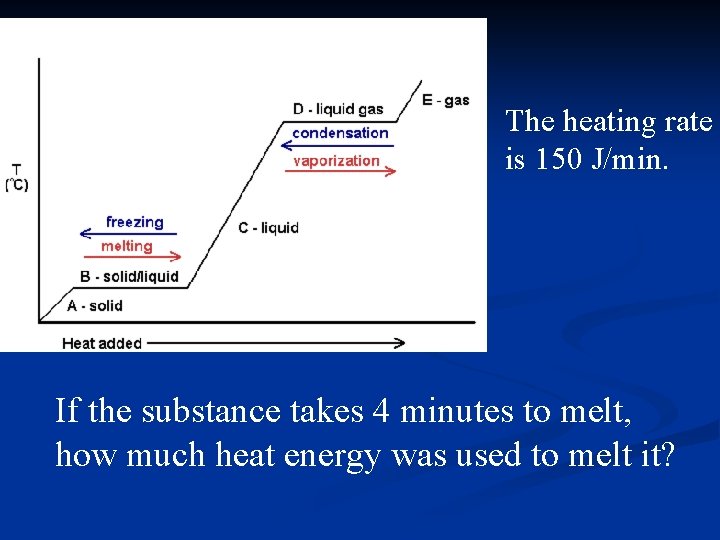
The heating rate is 150 J/min. If the substance takes 4 minutes to melt, how much heat energy was used to melt it?

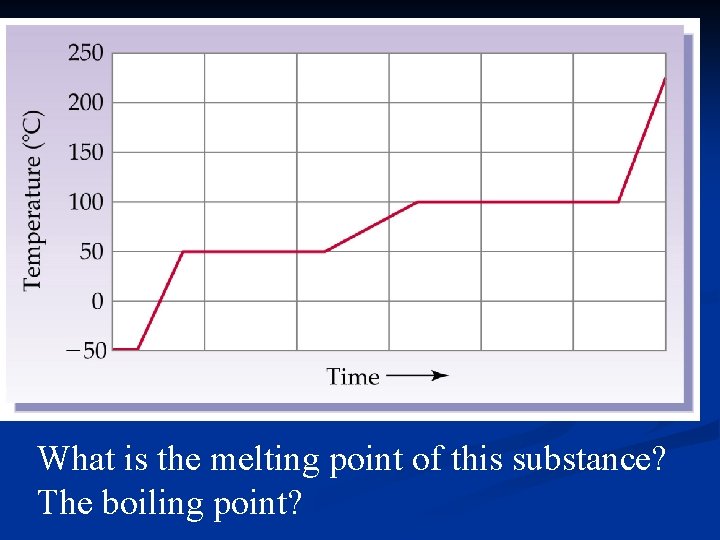
What is the melting point of this substance? The boiling point?

Heat of Fusion n Amount of energy required to change 1 gram of a pure substance from a solid to a liquid at its melting point. n Heat of Fusion = Hf n Hf = physical constant. for water = 333. 6 Joules per gram (Table B)

How much heat is absorbed when 10 grams of ice melts at 0 o. C? Heat absorbed = mass of substance X heat of fusion of substance n n Q = m. Hf = (10 g)(333. 6 J/g) = 3336 J n Where does that energy go? n Particles must overcome forces of attraction to move farther apart.


Heat of Vaporization n Amount of energy required to convert 1 gram of a pure substance from a liquid to a gas at its boiling point. n Heat of vaporization = constant n Hv Hv = physical for water = 2259 J/g
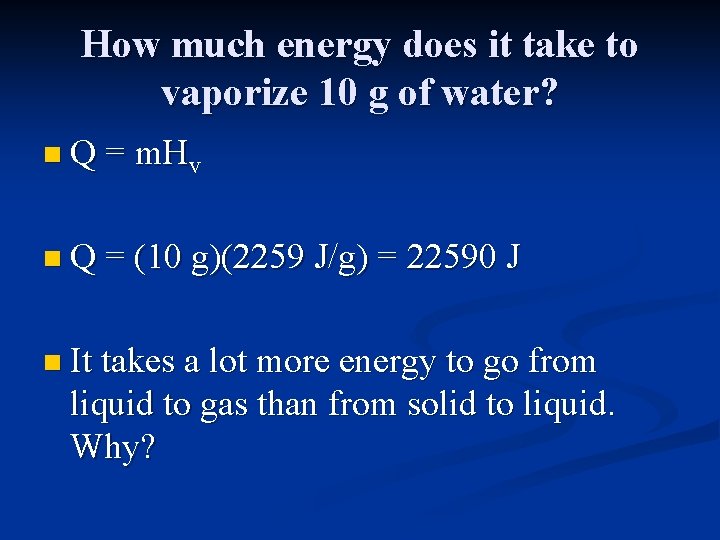
How much energy does it take to vaporize 10 g of water? n Q = m. Hv n Q = (10 g)(2259 J/g) = 22590 J n It takes a lot more energy to go from liquid to gas than from solid to liquid. Why?

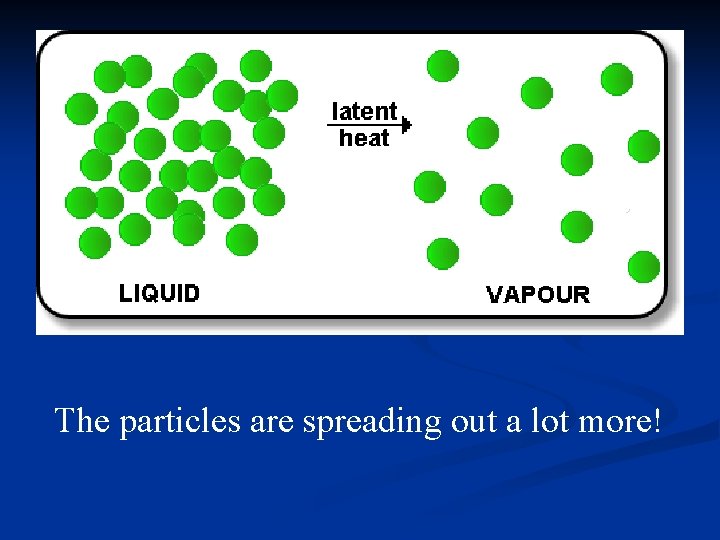
The particles are spreading out a lot more!

Heats of fusion & vaporization n Determined in calorimetry experiments.

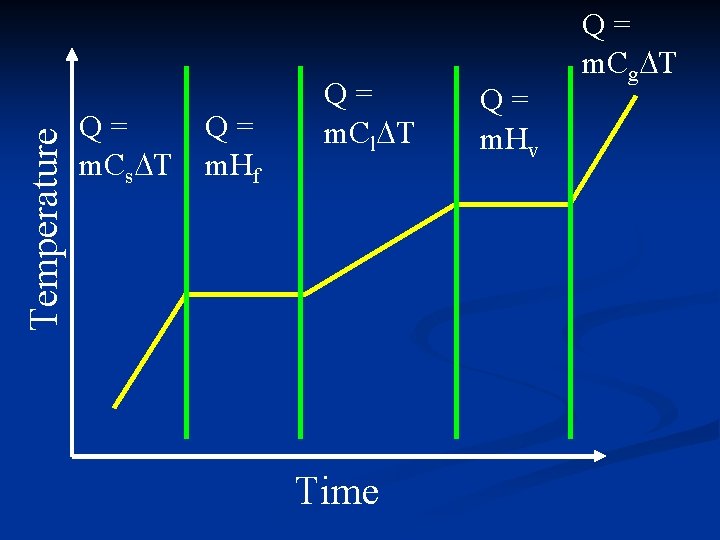
Temperature Q= m. Cs T Q= m. Hf Q= m. Cl T Time Q= m. Hv Q= m. Cg T

3 equations for Q n Q = m. C T n Q = m. Hf n Q = m. Hv n Have to figure out which one to use for a given problem. n Depends which section of heating curve. n Look for hints in the problem.

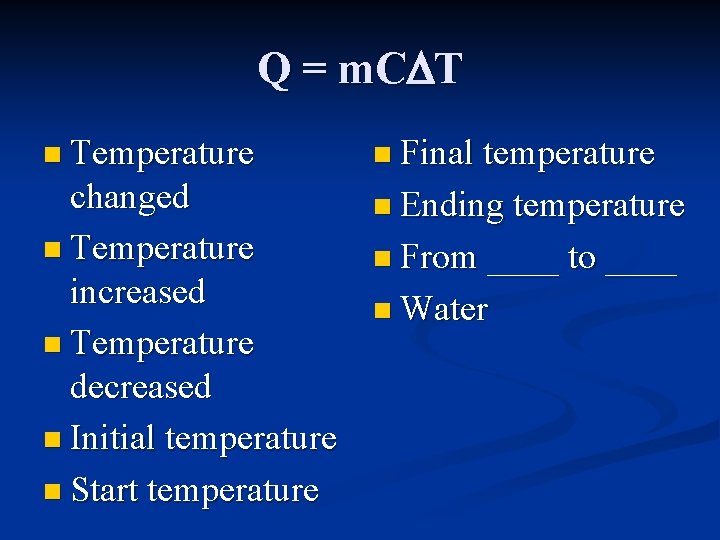
Q = m. C T n Temperature n Final temperature changed n Temperature increased n Temperature decreased n Initial temperature n Start temperature n Ending temperature n From ____ to ____ n Water

Q = m. Hf n Ice n Freezing n Melting n At 0 C (for H 2 O) n At constant temperature

Q = m. Hv n Steam n Boiling n Condensation n At 100 C (for H 2 O) n At constant temperature

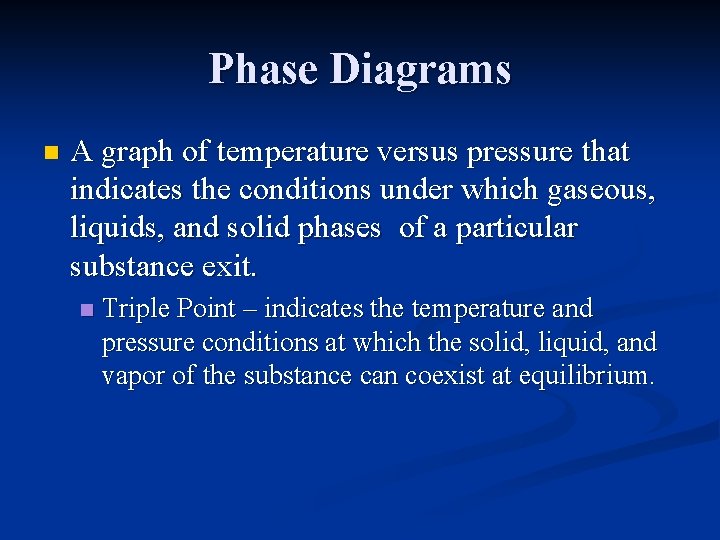
Phase Diagrams n A graph of temperature versus pressure that indicates the conditions under which gaseous, liquids, and solid phases of a particular substance exit. n Triple Point – indicates the temperature and pressure conditions at which the solid, liquid, and vapor of the substance can coexist at equilibrium.

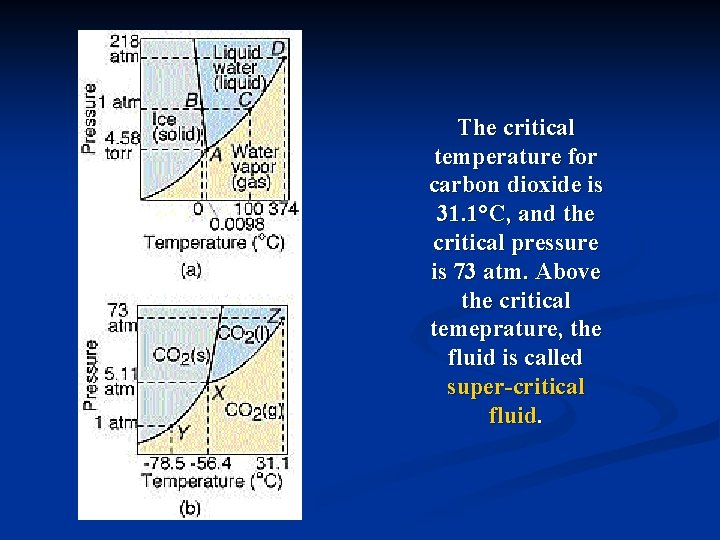
The critical temperature for carbon dioxide is 31. 1°C, and the critical pressure is 73 atm. Above the critical temeprature, the fluid is called super-critical fluid.