Heating Curves PHASE CHANGES ENERGY Phase Changes All


- Slides: 6

Heating Curves PHASE CHANGES & ENERGY

Phase Changes All phase changes occur at a constant temperature and have a constant kinetic energy To get to a phase change ◦ Heat added = increasing the movement of the particles = increasing the kinetic energy ◦ Heat released = decreasing the movement of the particles = decreasing the kinetic energy These changes in kinetic energy allow the molecules/atoms to move apart (increasing energy) or come together (decreasing energy)

Types of Phase Changes Fusion (melting): phase change from a solid to a liquid Boiling (vaporization): phase change from a liquid to a gas Sublimation: phase change from a solid to a gas Solidification (freezing): phase change from a liquid to a solid Condensation: phase change from a gas to a liquid Deposition: phase change from a gas to a solid Which 3 phase changes are exothermic? Which 3 are endothermic?

Heating Curves Heating curves: graphically describe the energy changes that take place during phase changes ◦ Show the temperature of each phase change ◦ Show the boiling and melting points of a substance ◦ Can be used to determine the phase of matter of a substance at a given temperature

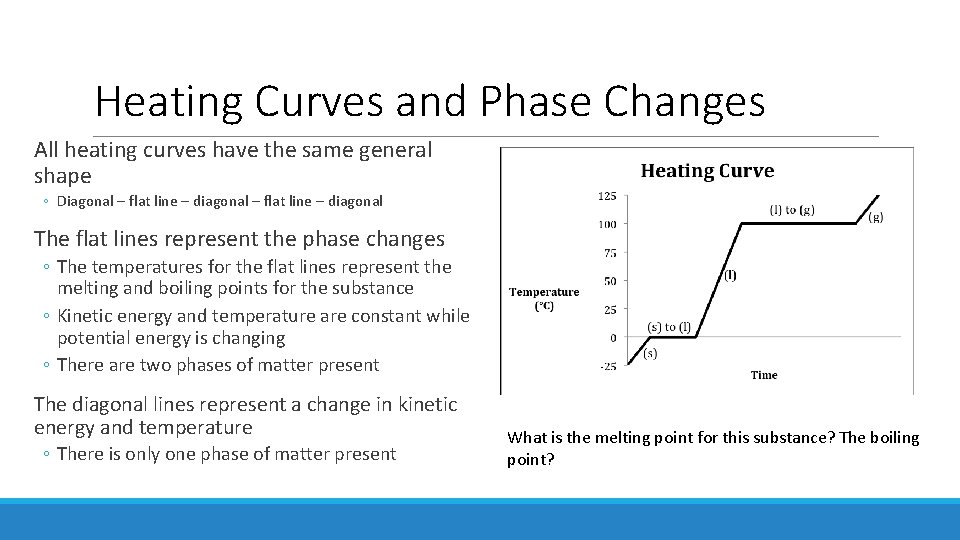
Heating Curves and Phase Changes All heating curves have the same general shape ◦ Diagonal – flat line – diagonal The flat lines represent the phase changes ◦ The temperatures for the flat lines represent the melting and boiling points for the substance ◦ Kinetic energy and temperature are constant while potential energy is changing ◦ There are two phases of matter present The diagonal lines represent a change in kinetic energy and temperature ◦ There is only one phase of matter present What is the melting point for this substance? The boiling point?

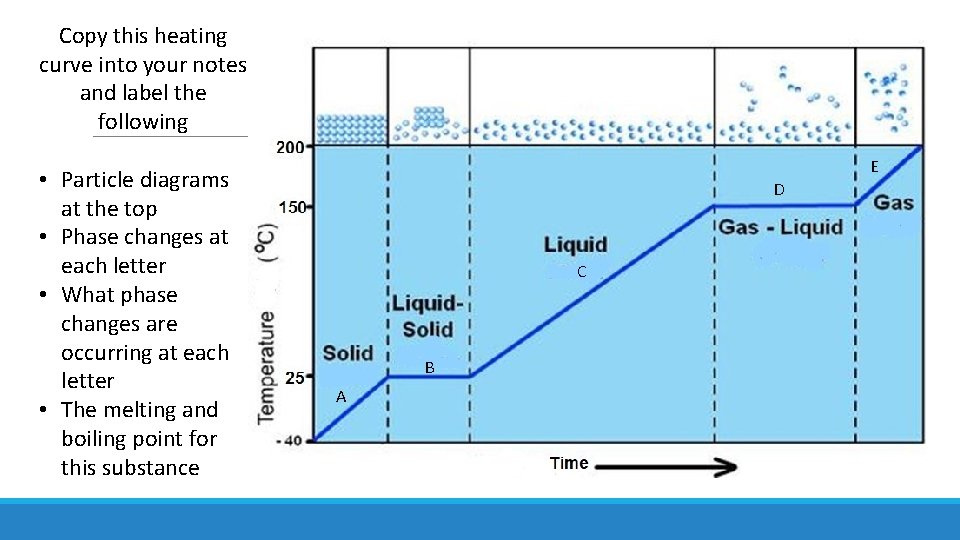
Copy this heating curve into your notes and label the following • Particle diagrams at the top • Phase changes at each letter • What phase changes are occurring at each letter • The melting and boiling point for this substance E D C B A