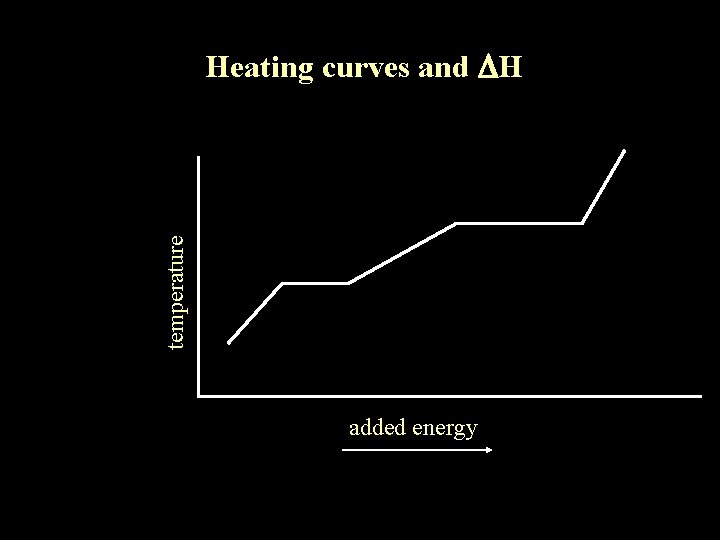
temperature Heating curves and DH added energy Heating

- Slides: 32

temperature Heating curves and DH added energy

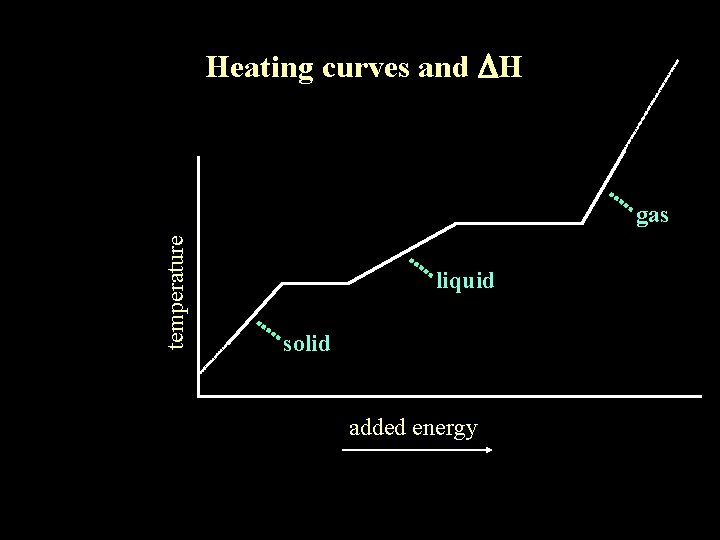
Heating curves and DH temperature gas liquid solid added energy

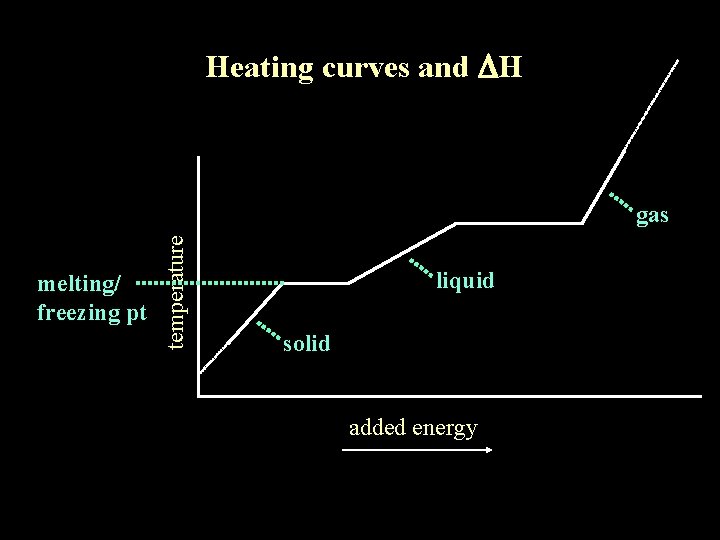
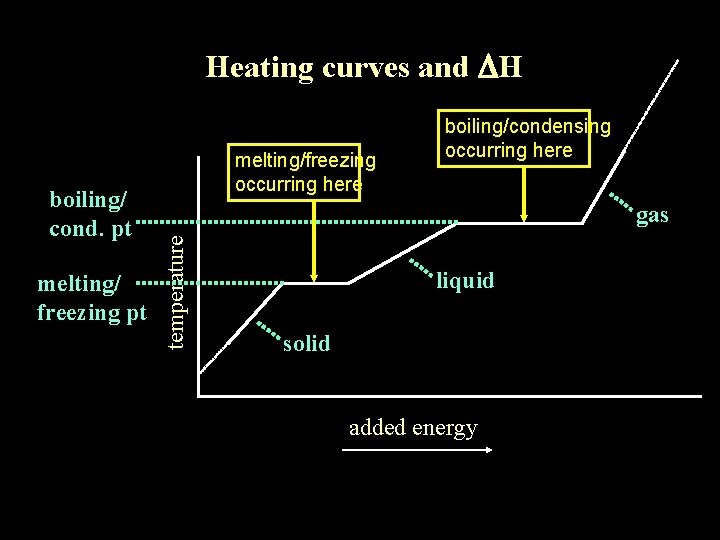
Heating curves and DH melting/ freezing pt temperature gas liquid solid added energy

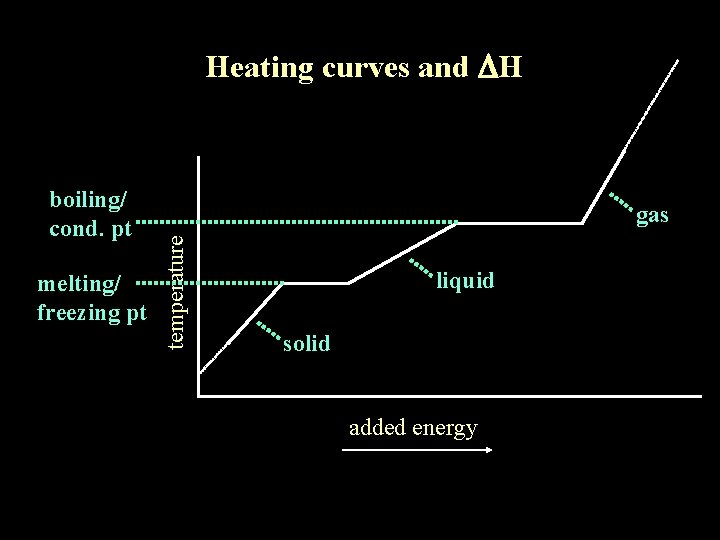
Heating curves and DH melting/ freezing pt gas temperature boiling/ cond. pt liquid solid added energy

Heating curves and DH melting/ freezing pt gas temperature boiling/ cond. pt melting/freezing occurring here boiling/condensing occurring here liquid solid added energy

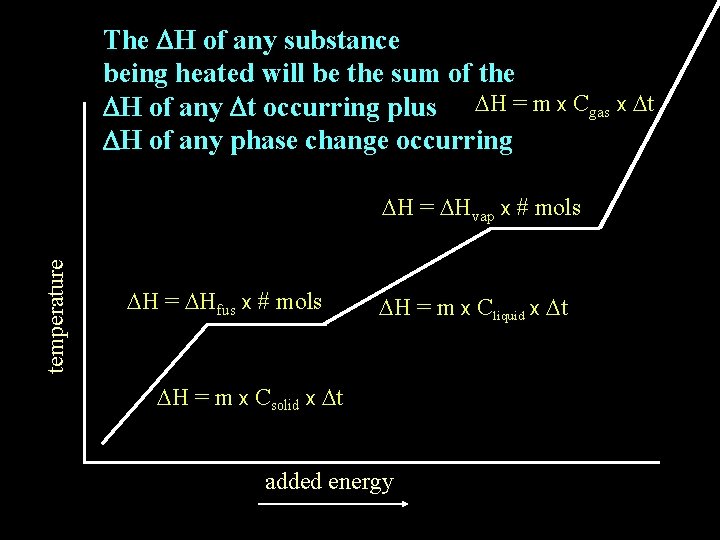
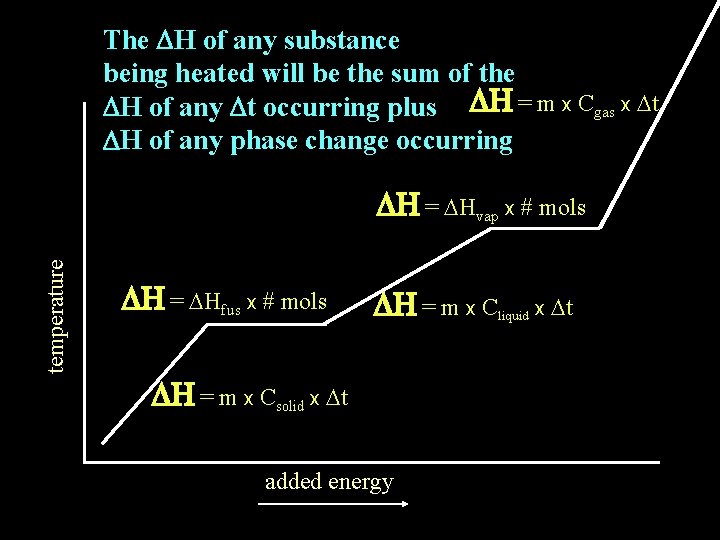
How is the total enthalpy change (DH) calculated for a substance whose temperature change includes a change in state?

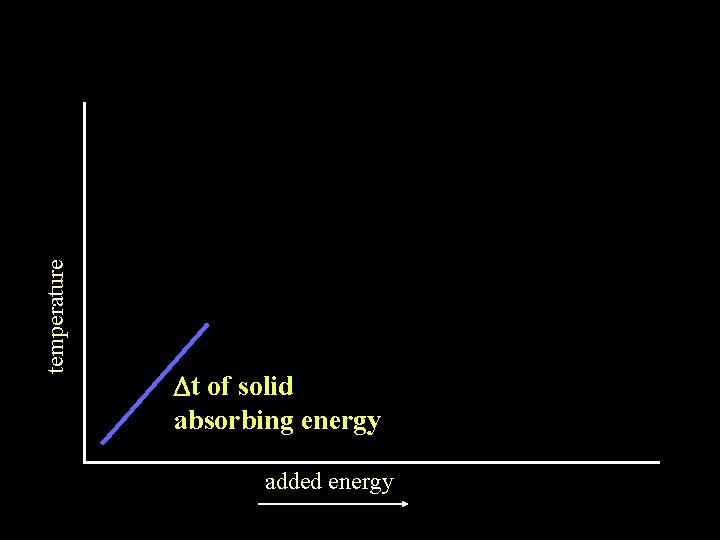
temperature Dt of solid absorbing energy added energy

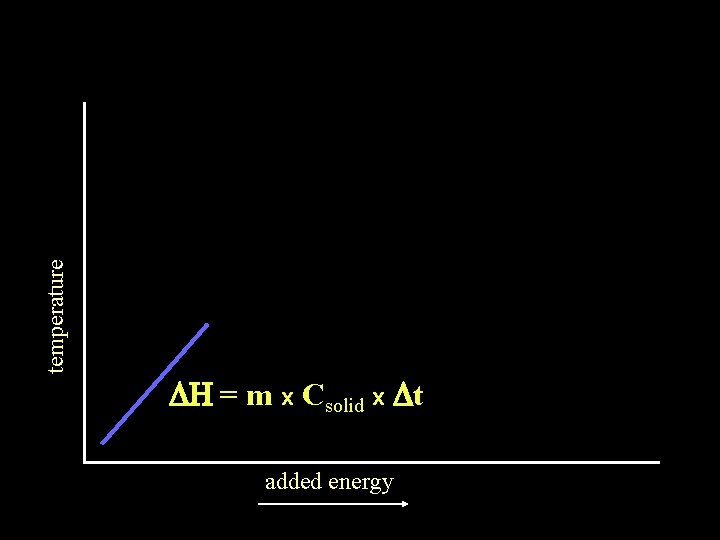
temperature DH = m x Csolid x Dt added energy

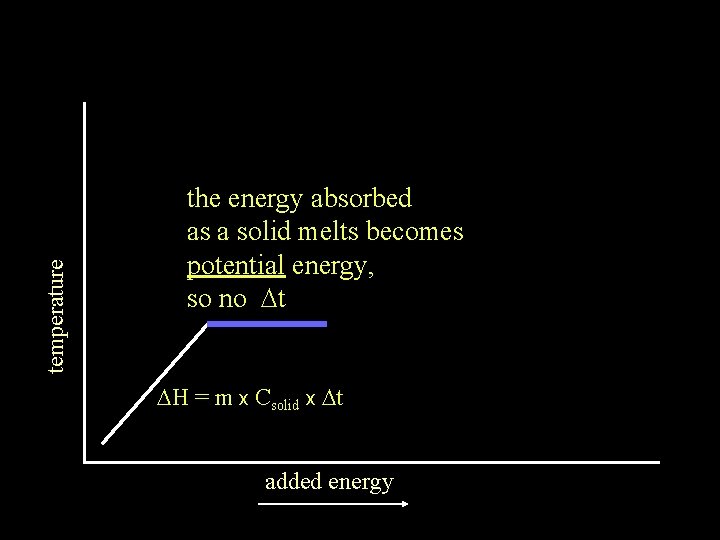
temperature the energy absorbed as a solid melts becomes potential energy, so no Dt DH = m x Csolid x Dt added energy

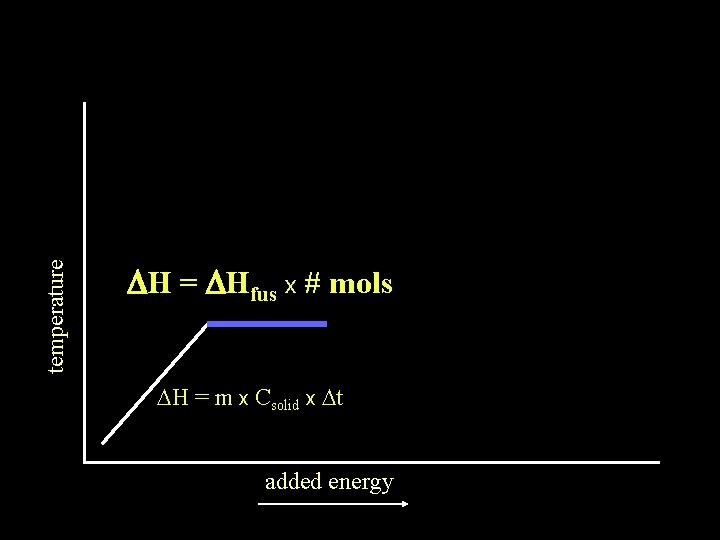
temperature DH = DHfus x # mols DH = m x Csolid x Dt added energy

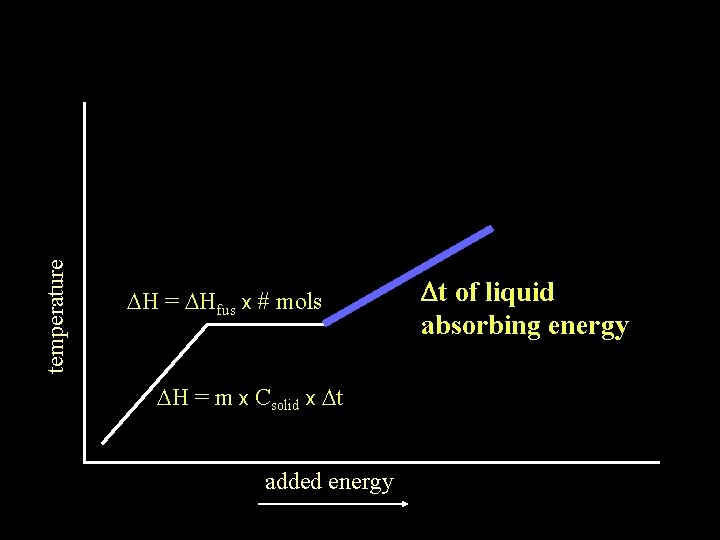
temperature DH = DHfus x # mols DH = m x Csolid x Dt added energy Dt of liquid absorbing energy

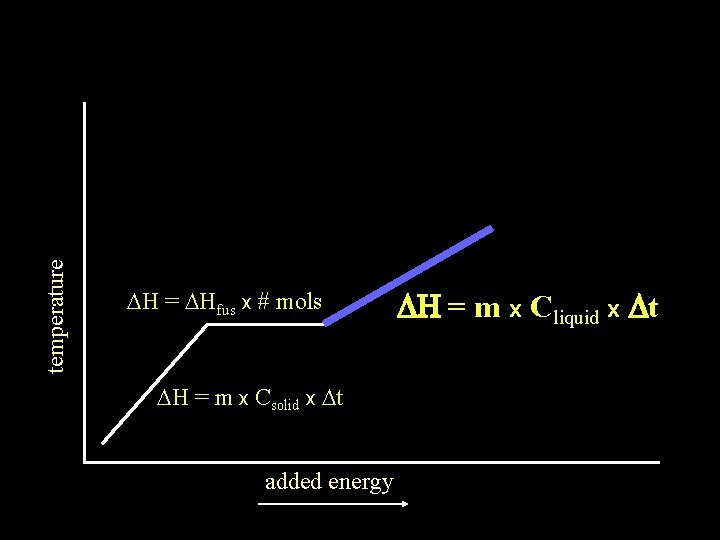
temperature DH = DHfus x # mols DH = m x Csolid x Dt added energy DH = m x Cliquid x Dt

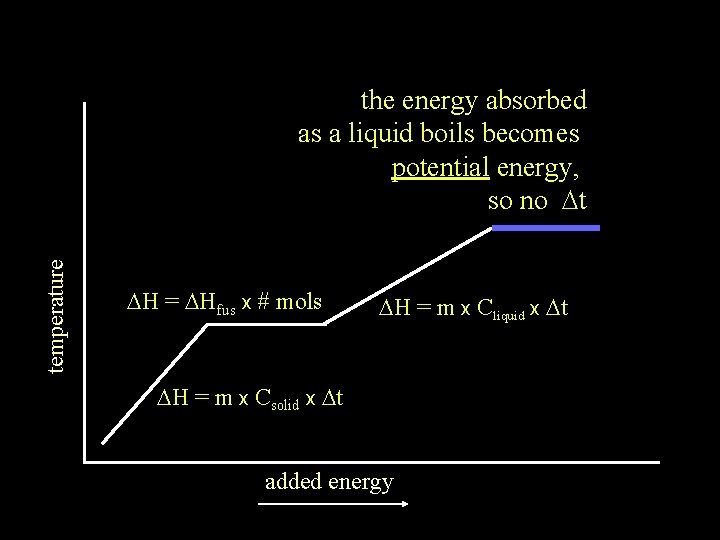
temperature the energy absorbed as a liquid boils becomes potential energy, so no Dt DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

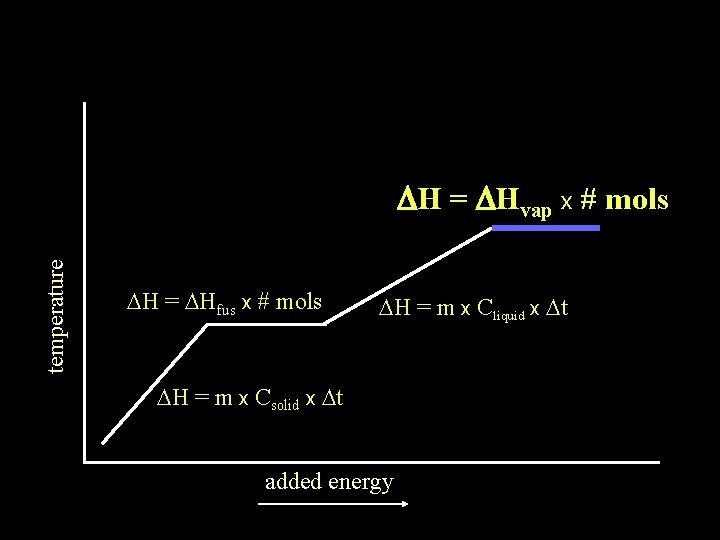
temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

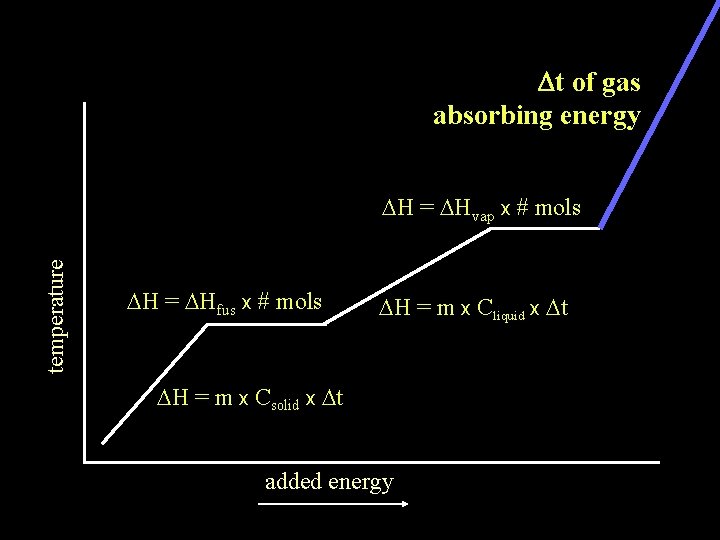
Dt of gas absorbing energy temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

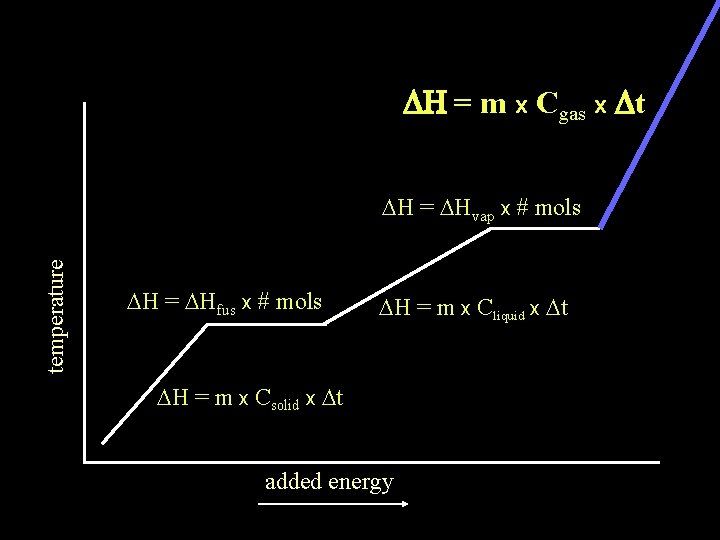
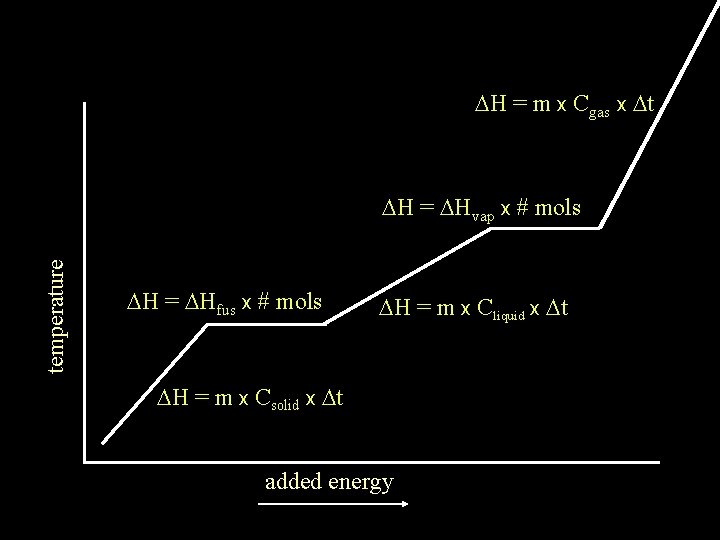
DH = m x Cgas x Dt temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

DH = m x Cgas x Dt temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

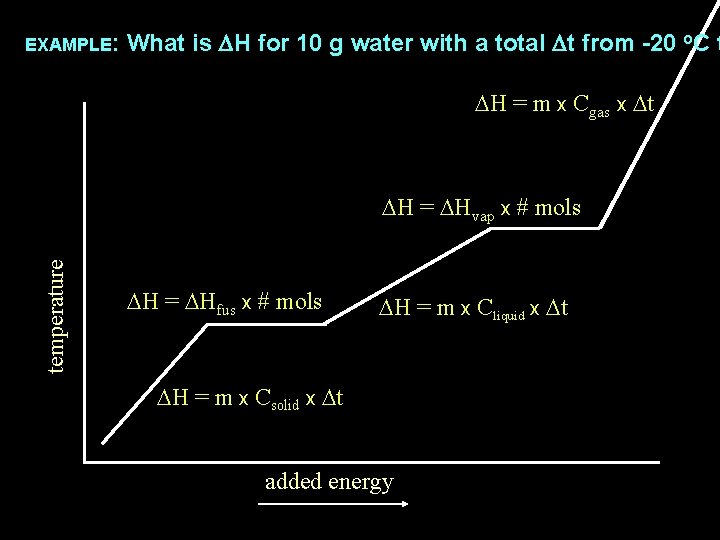
The DH of any substance being heated will be the sum of the DH of any Dt occurring plus DH = m x Cgas x Dt DH of any phase change occurring temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

The DH of any substance being heated will be the sum of the DH of any Dt occurring plus DH = m x Cgas x Dt DH of any phase change occurring temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

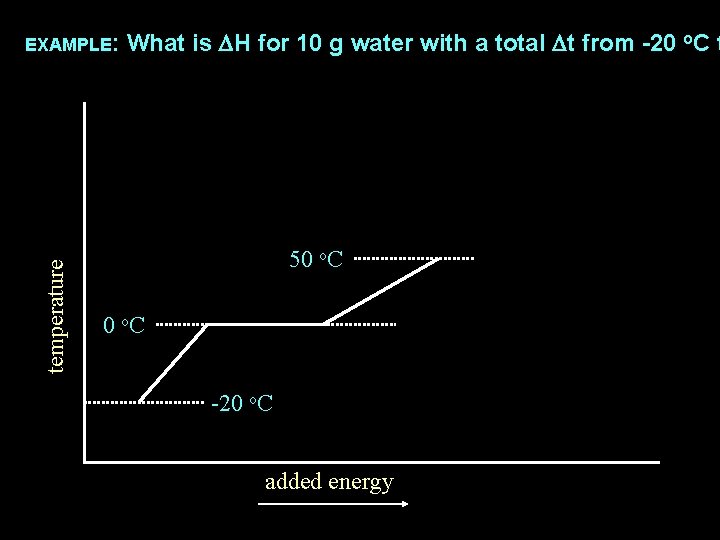
EXAMPLE: What is DH for 10 g water with a total Dt from -20 o. C t DH = m x Cgas x Dt temperature DH = DHvap x # mols DH = DHfus x # mols DH = m x Cliquid x Dt DH = m x Csolid x Dt added energy

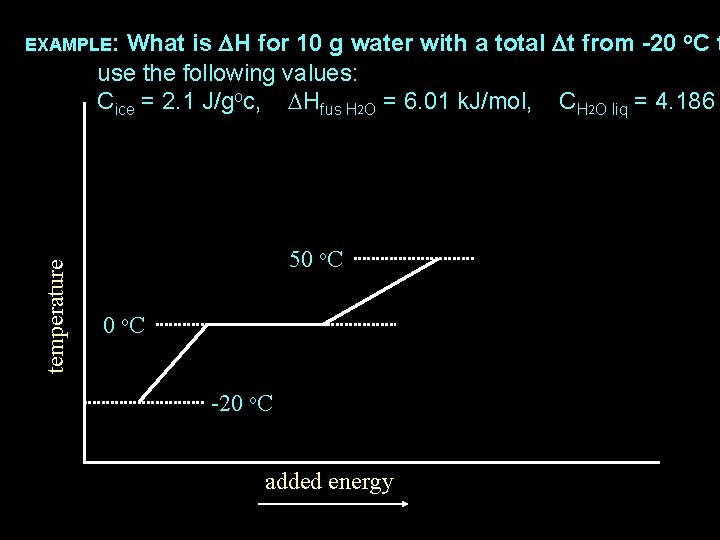
temperature EXAMPLE: What is DH for 10 g water with a total Dt from -20 o. C t 50 o. C -20 o. C added energy

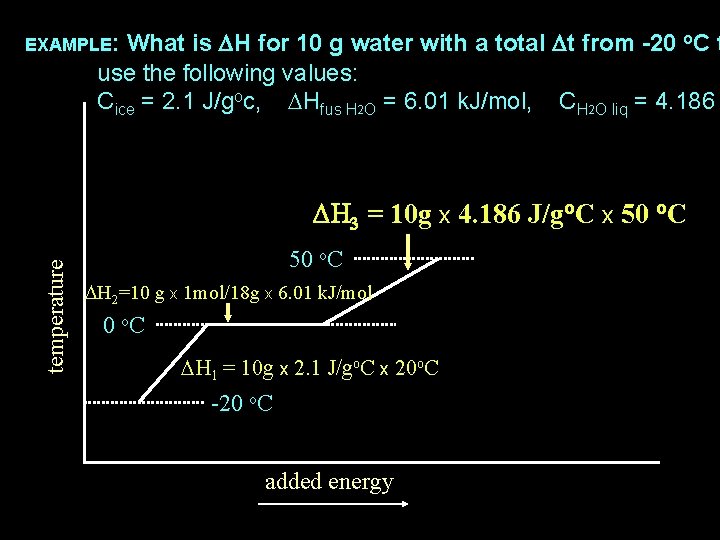
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 temperature EXAMPLE: 50 o. C -20 o. C added energy

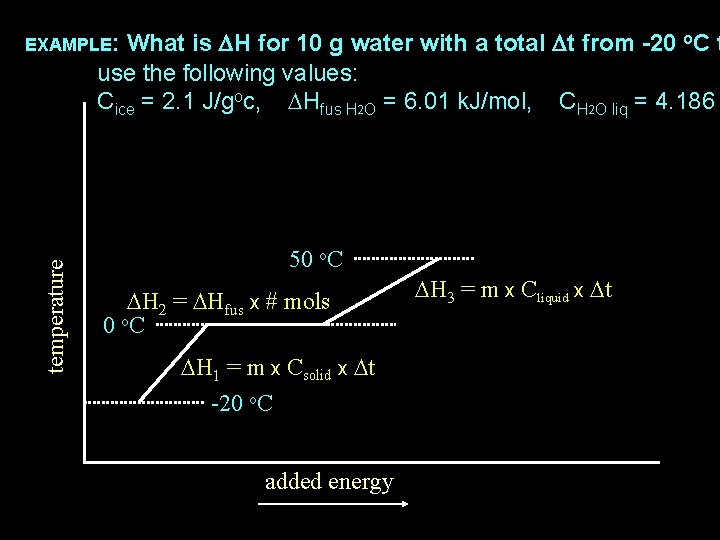
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 temperature EXAMPLE: 50 o. C DH 2 = DHfus x # mols 0 o. C DH 1 = m x Csolid x Dt -20 o. C added energy DH 3 = m x Cliquid x Dt

What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 temperature EXAMPLE: 50 o. C DH 2 = DHfus x # mols 0 o. C DH 3 = m x Cliquid x Dt DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

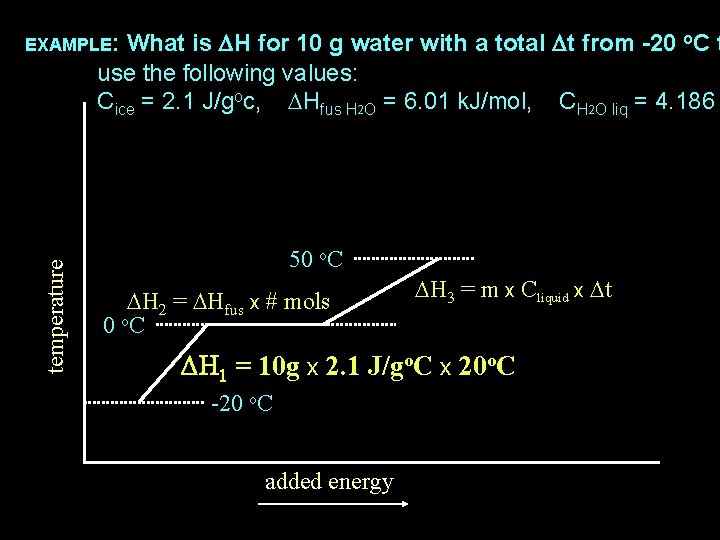
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 temperature EXAMPLE: 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol DH 3 = m x Cliquid x Dt 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

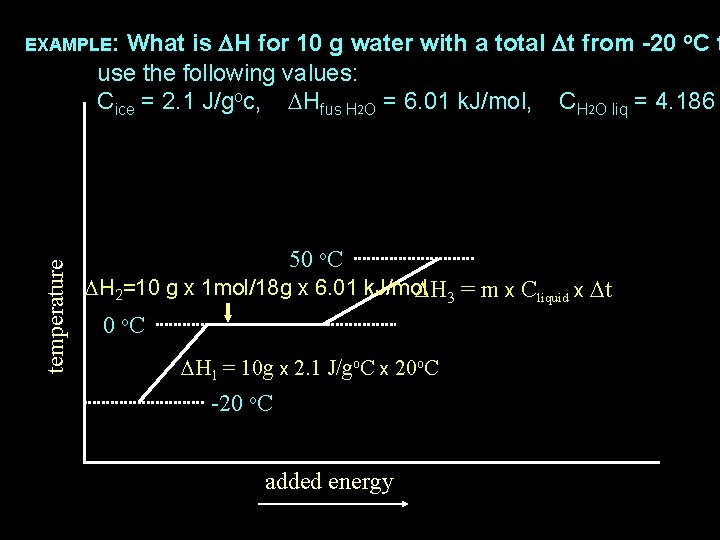
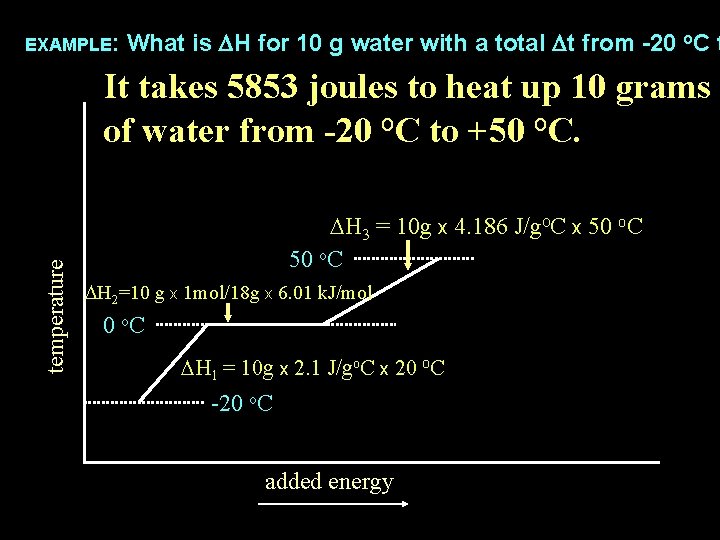
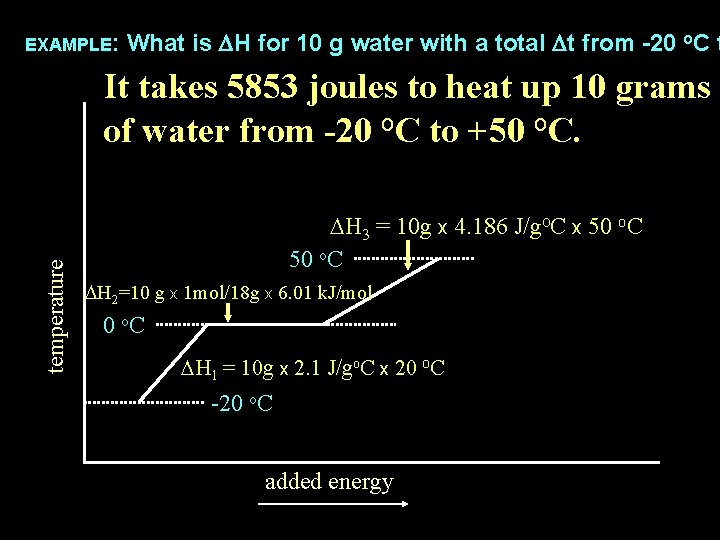
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 EXAMPLE: temperature DH 3 = 10 g x 4. 186 J/go. C x 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

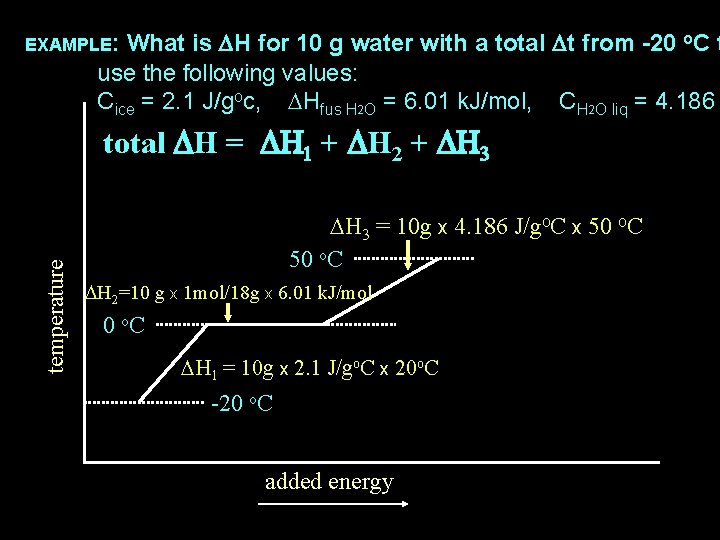
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 EXAMPLE: temperature total DH = DH 1 + DH 2 + DH 3 = 10 g x 4. 186 J/go. C x 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

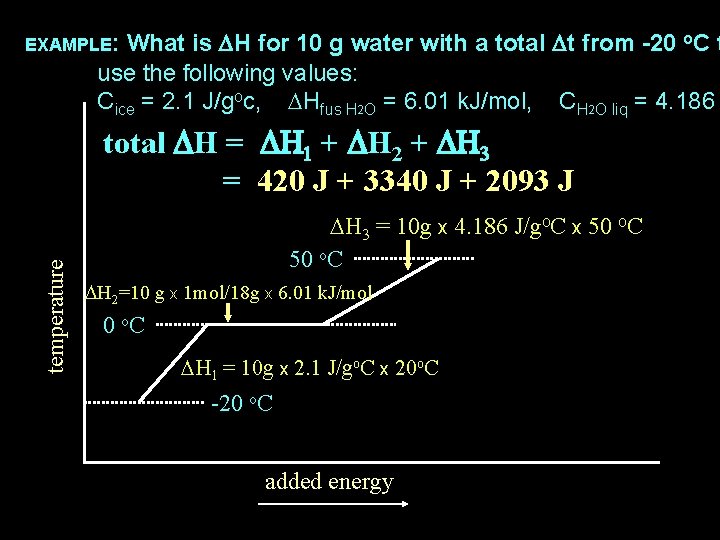
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 EXAMPLE: temperature total DH = DH 1 + DH 2 + DH 3 = 420 J + 3340 J + 2093 J DH 3 = 10 g x 4. 186 J/go. C x 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

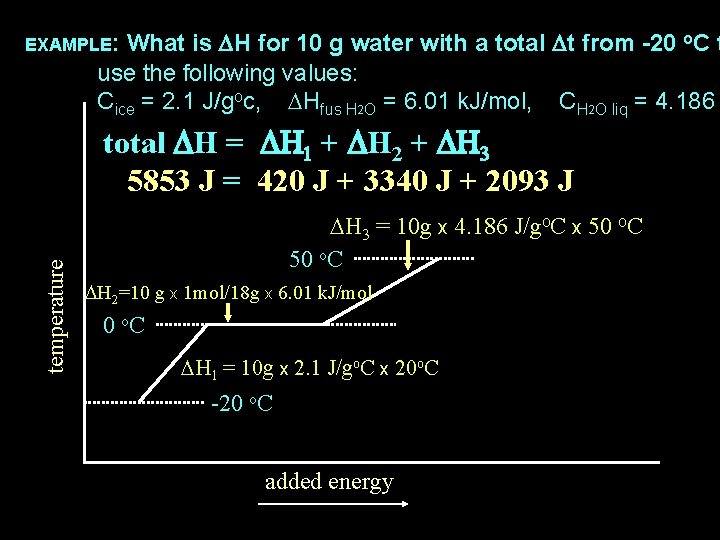
What is DH for 10 g water with a total Dt from -20 o. C t use the following values: Cice = 2. 1 J/goc, DHfus H 2 O = 6. 01 k. J/mol, CH 2 O liq = 4. 186 EXAMPLE: temperature total DH = DH 1 + DH 2 + DH 3 5853 J = 420 J + 3340 J + 2093 J DH 3 = 10 g x 4. 186 J/go. C x 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

EXAMPLE: What is DH for 10 g water with a total Dt from -20 o. C t temperature It takes 5853 joules to heat up 10 grams of water from -20 o. C to +50 o. C. DH 3 = 10 g x 4. 186 J/go. C x 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

EXAMPLE: What is DH for 10 g water with a total Dt from -20 o. C t temperature It takes 5853 joules to heat up 10 grams of water from -20 o. C to +50 o. C. DH 3 = 10 g x 4. 186 J/go. C x 50 o. C DH 2=10 g x 1 mol/18 g x 6. 01 k. J/mol 0 o. C DH 1 = 10 g x 2. 1 J/go. C x 20 o. C -20 o. C added energy

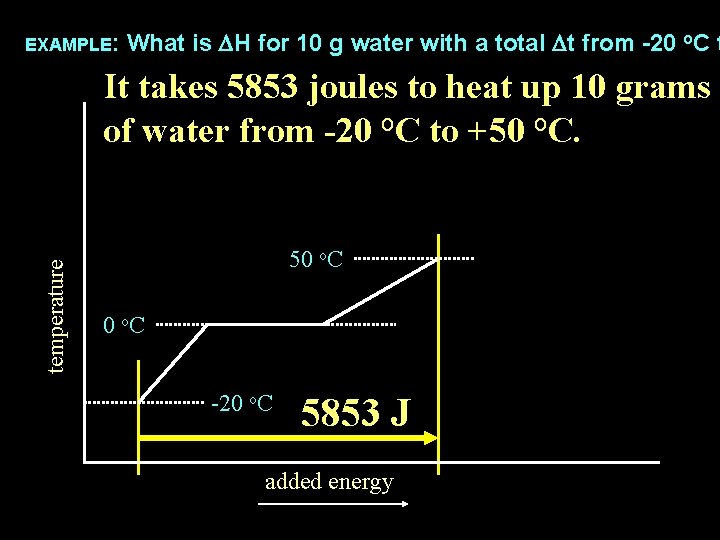
EXAMPLE: What is DH for 10 g water with a total Dt from -20 o. C t temperature It takes 5853 joules to heat up 10 grams of water from -20 o. C to +50 o. C. 50 o. C -20 o. C 5853 J added energy