Calcite Vaterite Aragonite Solid Phases Calcite most common














- Slides: 19

Calcite Vaterite Aragonite

Solid Phases • Calcite - most common and stable • Aragonite - often kinetically favored • Vaterite - rare in nature


Potential Causes of Whitings • • Increased temperature Increased Ca 2+ and/or CO 32 phytoplankton blooms combination of all of above

Background: Nucleation • Homogeneous nucleation - from solution • Heterogeneous Nucleation - on a substrate • Induction time

Background: Saturation State • Often denoted by W • = (a. Ca 2+a. CO 32 -)/Karagonite • If W > 1 for a mineral in solution, precipitation is favored

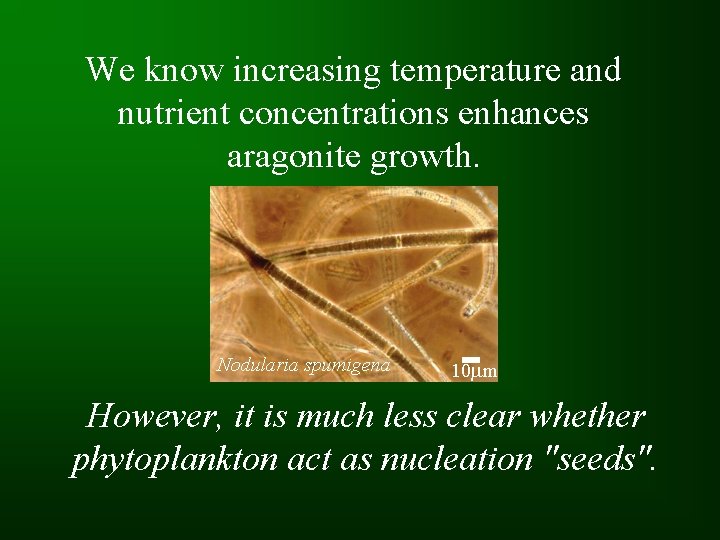
We know increasing temperature and nutrient concentrations enhances aragonite growth. Nodularia spumigena 10 mm However, it is much less clear whether phytoplankton act as nucleation "seeds".

Simple Nucleation Experiments • Add excess Ca 2+ to Pyramid Lake water until whiting is observed. • Quantify how much Ca 2+ is required for given conditions

Equipment Required • • • Collection bottle Filtration apparatus Clock or watch Stir plate Burette • • • Glass flask 0. 1 M Ca. Cl 2 solution Thermometer Notebook and pen p. H meter (optional)

Variables to Keep Constant • • Temperature Stir rate Interval of Ca 2+ additions Particulates in solution

Procedure • Add drops of 0. 1 M Ca. Cl 2 solution in predetermined time intervals • Record time and p. H after each addition • Stop when whiting is observed

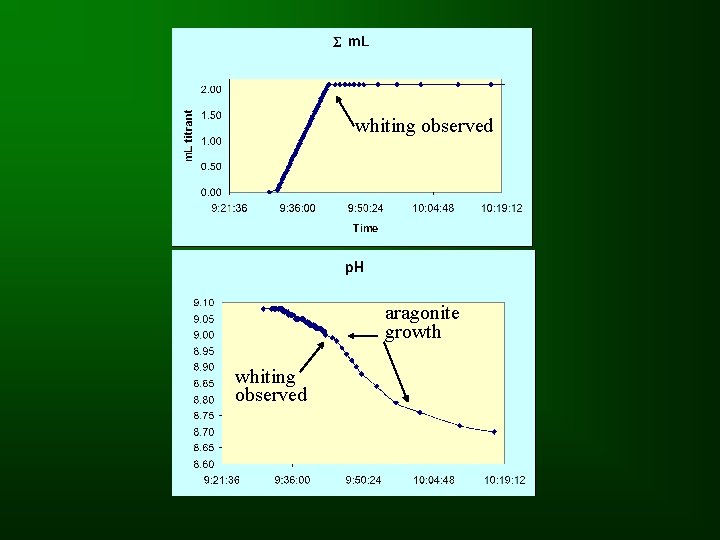
Data • The main variable of interest is the amount of titrant required to produce the whiting event.

whiting observed aragonite growth whiting observed

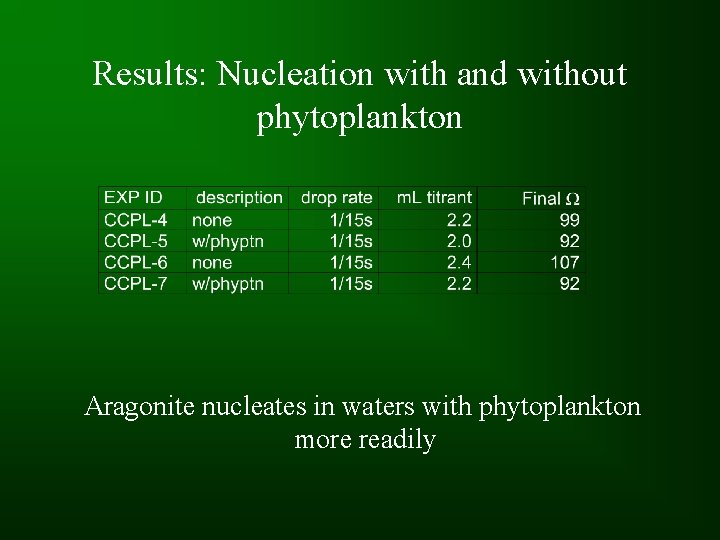
Results: Nucleation with and without phytoplankton Aragonite nucleates in waters with phytoplankton more readily

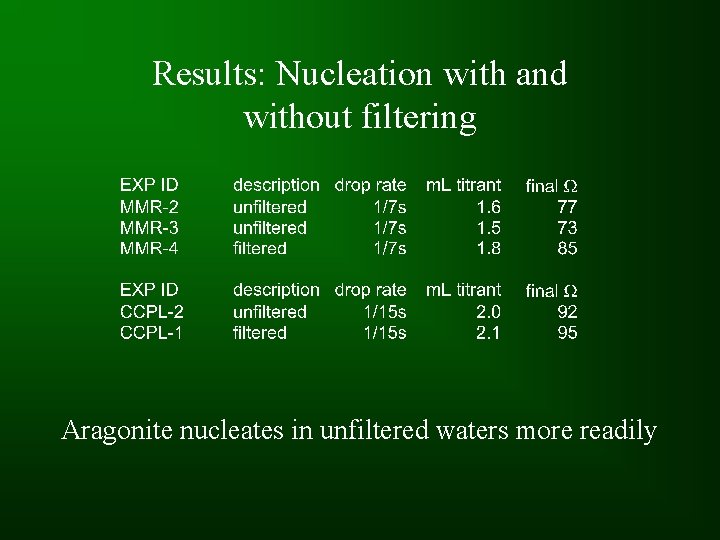
Results: Nucleation with and without filtering Aragonite nucleates in unfiltered waters more readily

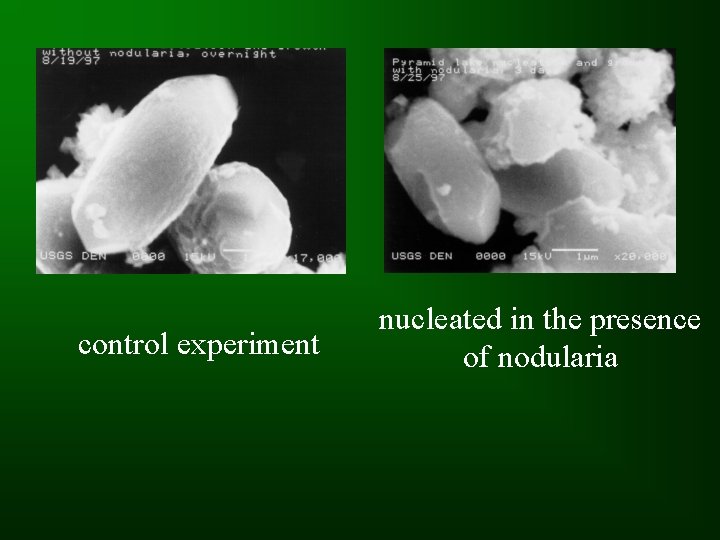
SEM Imaging • Aragonite crystals look similar in experiments performed with and without added phytoplankton.

control experiment nucleated in the presence of nodularia

Discussion/Conclusions • The amount of added Ca 2+ required for nucleation was reproducible in control exps. • Filtering appears to have an effect on nucleation • Nucleation occurs more readily in the presence of phytoplankton; suggesting that algal blooms may enhance whiting events.

General implications for natural systems • Natural waters are commonly supersaturated with respect to Ca. CO 3 minerals, but the concentration and chemical nature of natural inhibitors will determine whether, and at what rate crystal growth actually occurs. • Substances that enhance nucleation of crystals do not necessarily affect mechanisms of growth, as these are two very different processes.