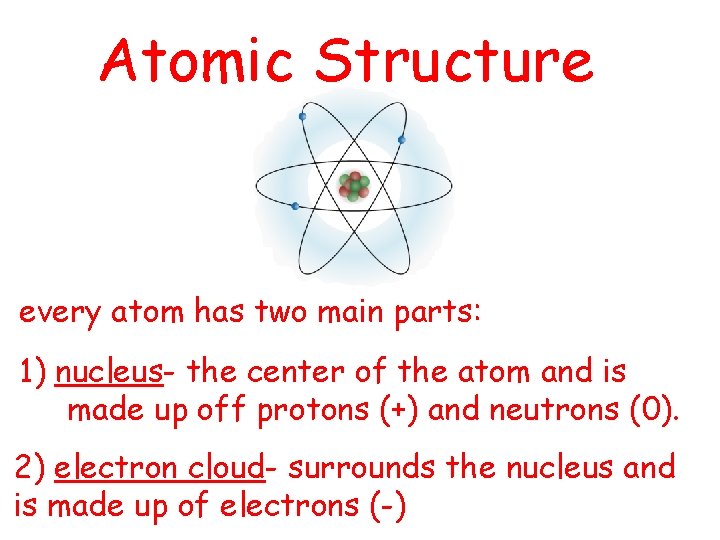
Atomic Structure every atom has two main parts

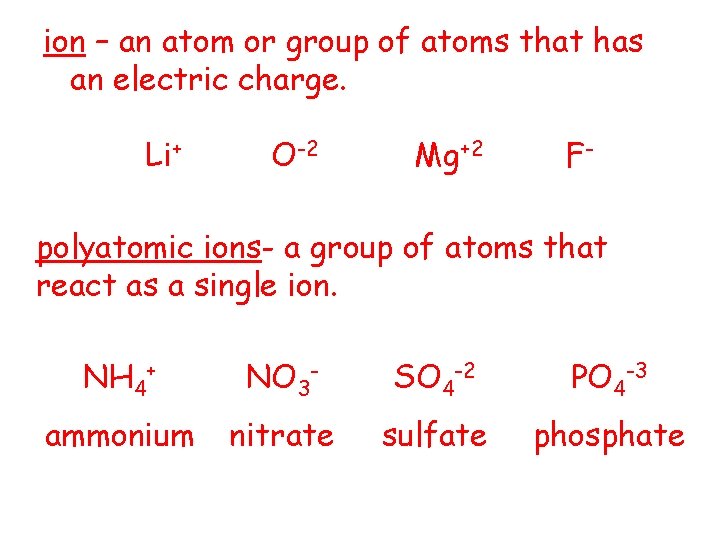

- Slides: 21

Atomic Structure every atom has two main parts: 1) nucleus- the center of the atom and is made up off protons (+) and neutrons (0). 2) electron cloud- surrounds the nucleus and is made up of electrons (-)

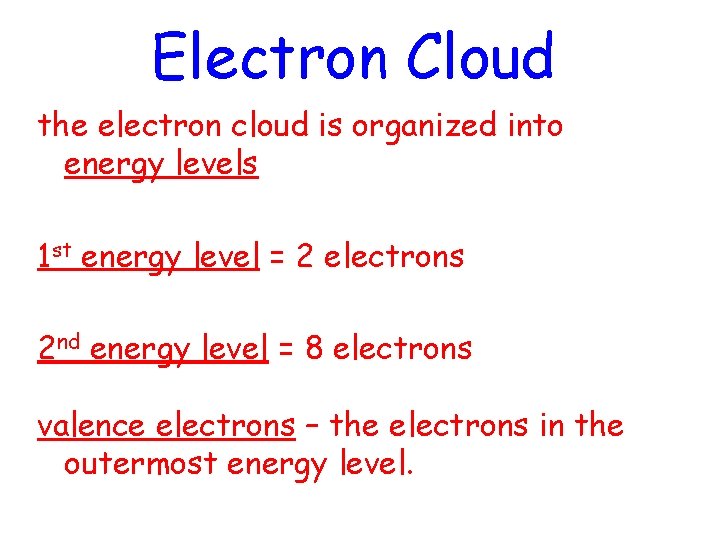
Electron Cloud the electron cloud is organized into energy levels 1 st energy level = 2 electrons 2 nd energy level = 8 electrons valence electrons – the electrons in the outermost energy level.

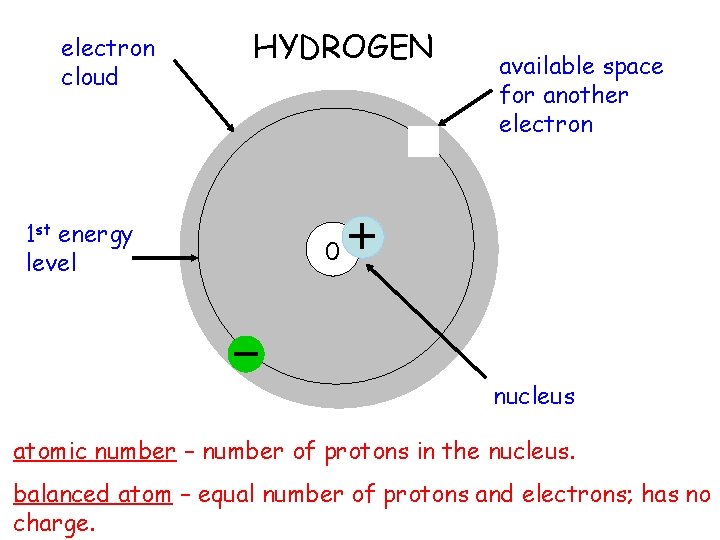
electron cloud 1 st energy level HYDROGEN available space for another electron 0 nucleus atomic number – number of protons in the nucleus. balanced atom – equal number of protons and electrons; has no charge.

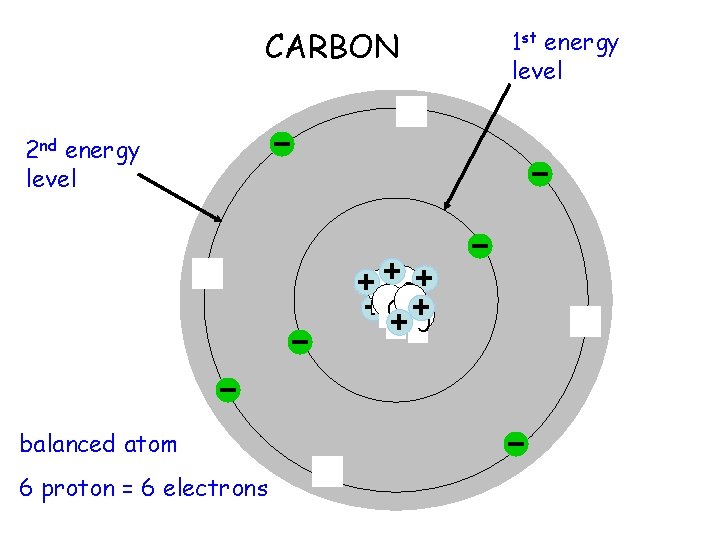
CARBON 2 nd energy level 00 0 balanced atom 6 proton = 6 electrons 1 st energy level

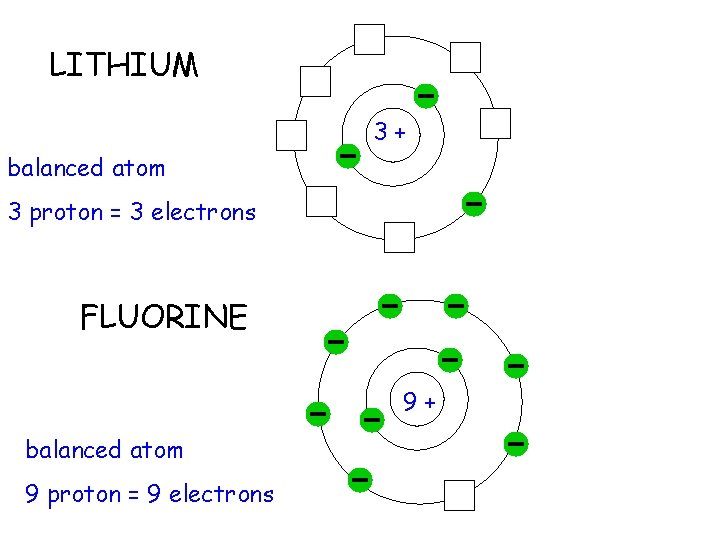
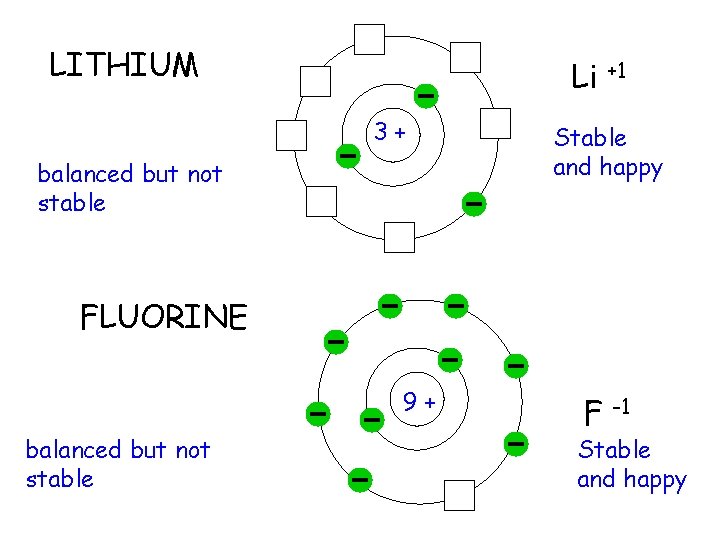
LITHIUM 3+ balanced atom 3 proton = 3 electrons FLUORINE 9+ balanced atom 9 proton = 9 electrons

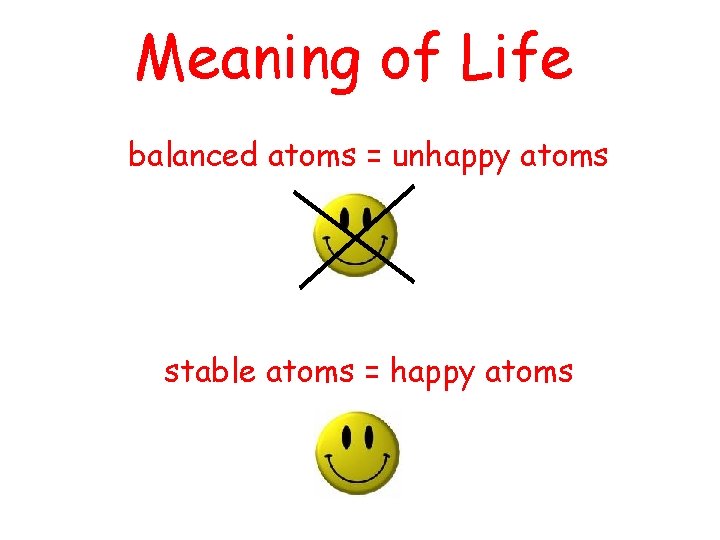
Meaning of Life balanced atoms = unhappy atoms stable atoms = happy atoms

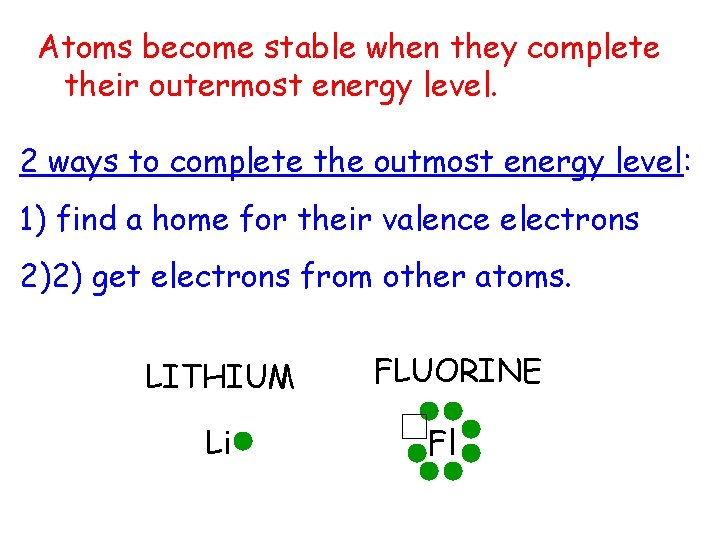
Atoms become stable when they complete their outermost energy level. 2 ways to complete the outmost energy level: 1) find a home for their valence electrons 2)2) get electrons from other atoms. LITHIUM Li FLUORINE Fl

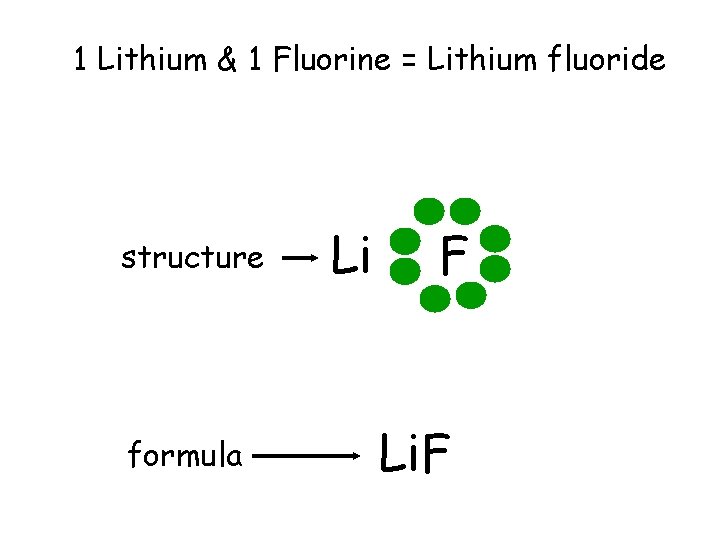
1 Lithium & 1 Fluorine = Lithium fluoride structure formula Li F Li. F

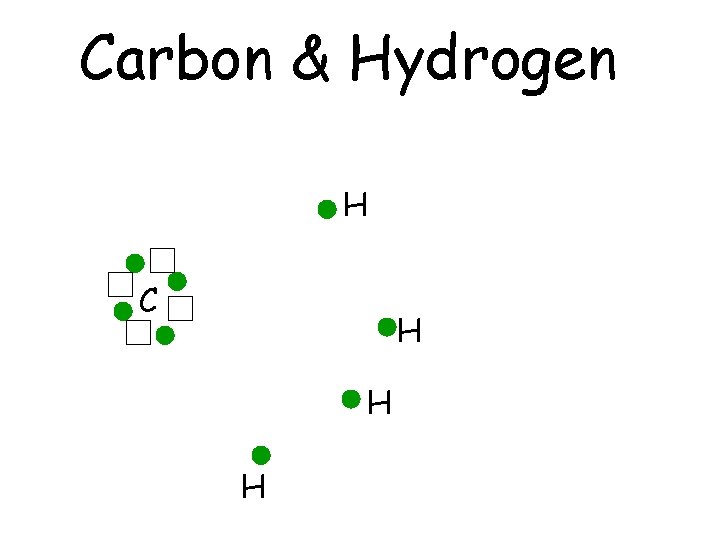
Carbon & Hydrogen H C H H H

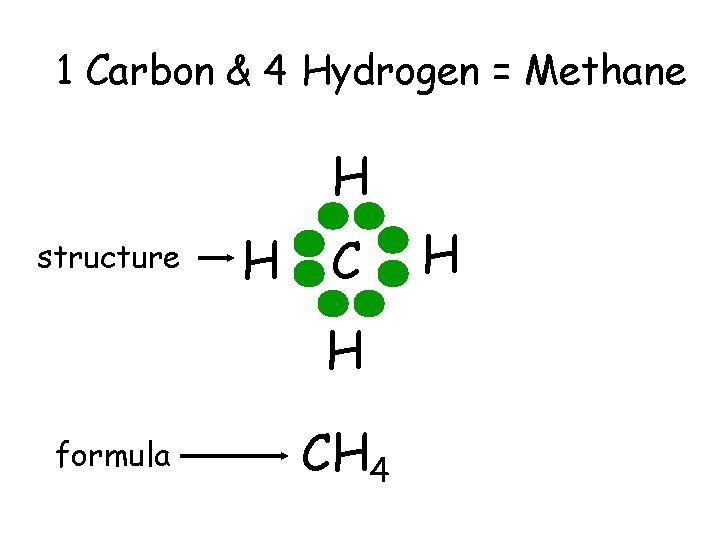
1 Carbon & 4 Hydrogen = Methane H structure H C H formula CH 4 H

Oxidation Numbers The oxidations number indicates if an atom is going to give up electrons or take in electrons in order to become stable. Positive oxidation numbers mean the atom is going to give up electrons. Negative oxidation numbers mean the atom is going to take in electrons

LITHIUM Li +1 3+ balanced but not stable Stable and happy FLUORINE 9+ balanced but not stable F -1 Stable and happy

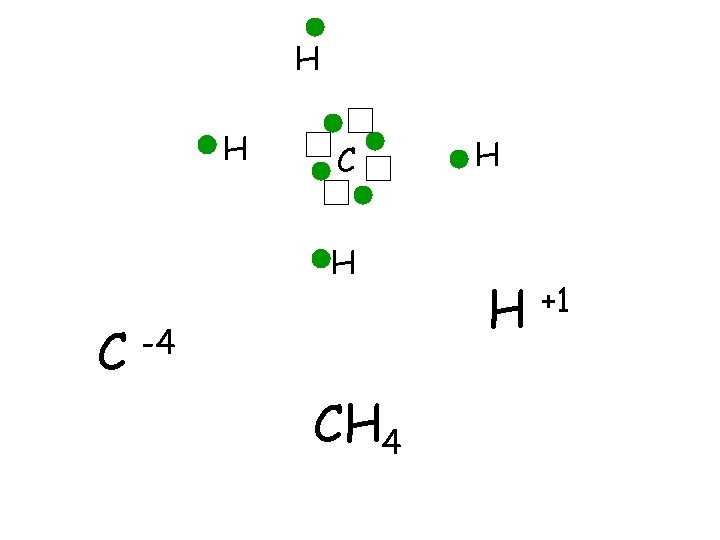
H H C -4 CH 4 H H +1

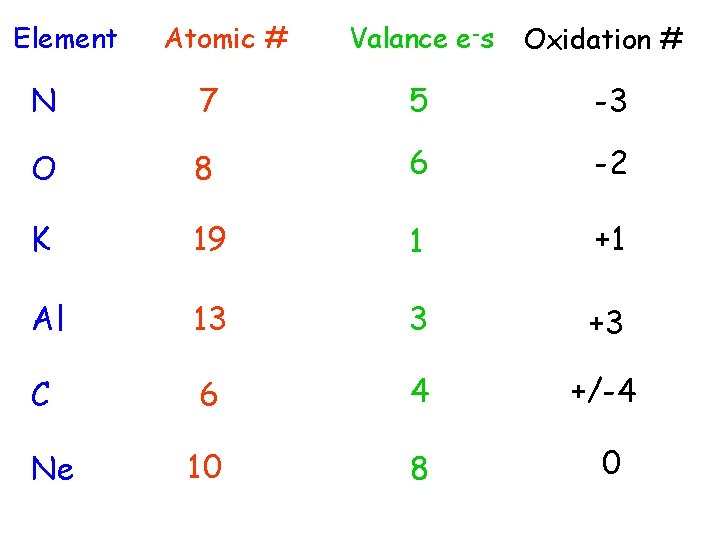
Element Atomic # Valance e-s Oxidation # N 7 5 -3 O 8 6 -2 K 19 1 +1 Al 13 3 +3 C 6 4 +/-4 10 8 0 Ne
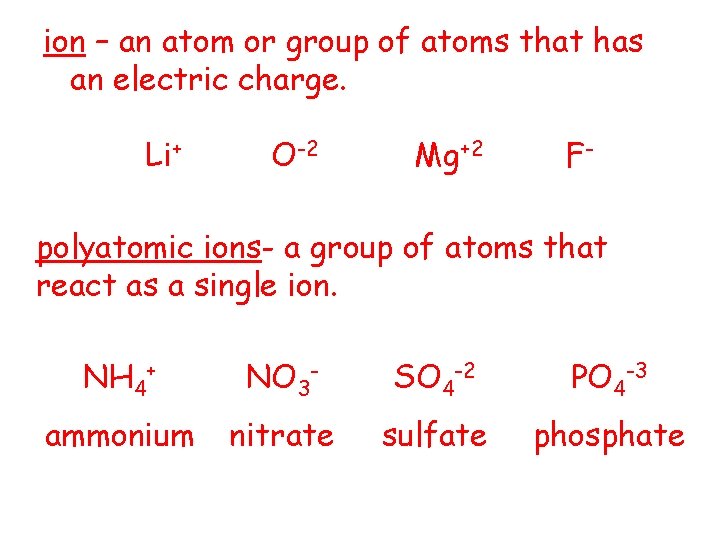
ion – an atom or group of atoms that has an electric charge. Li+ O-2 Mg+2 F- polyatomic ions- a group of atoms that react as a single ion. NH 4+ NO 3 - SO 4 -2 PO 4 -3 ammonium nitrate sulfate phosphate

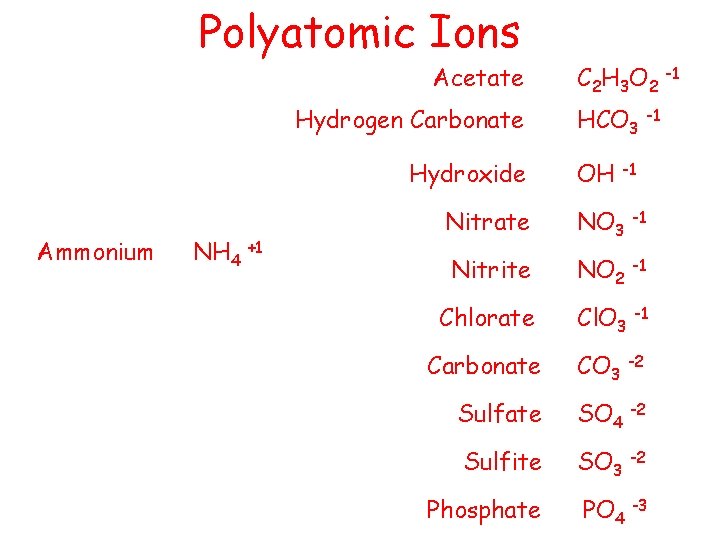
Polyatomic Ions Acetate Hydrogen Carbonate Hydroxide Ammonium NH 4 +1 C 2 H 3 O 2 -1 HCO 3 -1 OH -1 Nitrate NO 3 -1 Nitrite NO 2 -1 Chlorate Cl. O 3 -1 Carbonate CO 3 -2 Sulfate SO 4 -2 Sulfite SO 3 -2 Phosphate PO 4 -3

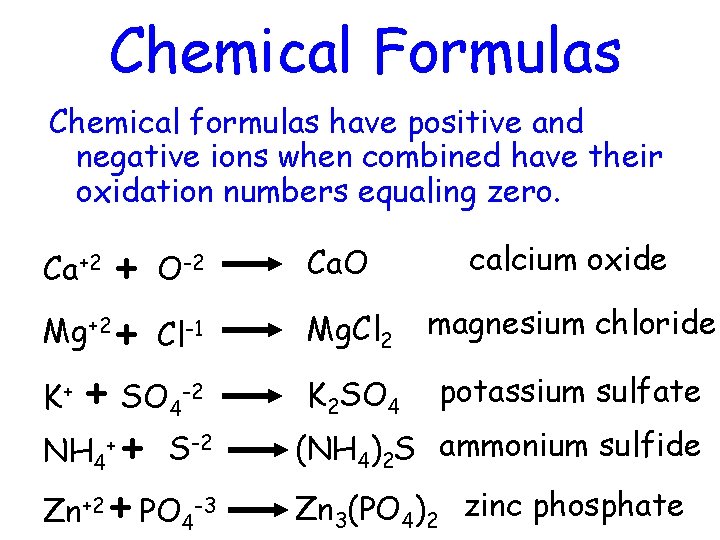
Chemical Formulas Chemical formulas have positive and negative ions when combined have their oxidation numbers equaling zero. + Mg+2 + Cl-1 K+ + SO 4 -2 NH 4+ + S-2 Zn+2 + PO 4 -3 Ca+2 O-2 Ca. O calcium oxide Mg. Cl 2 magnesium chloride K 2 SO 4 potassium sulfate (NH 4)2 S ammonium sulfide Zn 3(PO 4)2 zinc phosphate

Atoms become stable when they react with other atoms. When atoms react, they form a chemical bond. 3 Types of Chemical Bonds: 1) Ionic 2) Covalent 3) Metallic

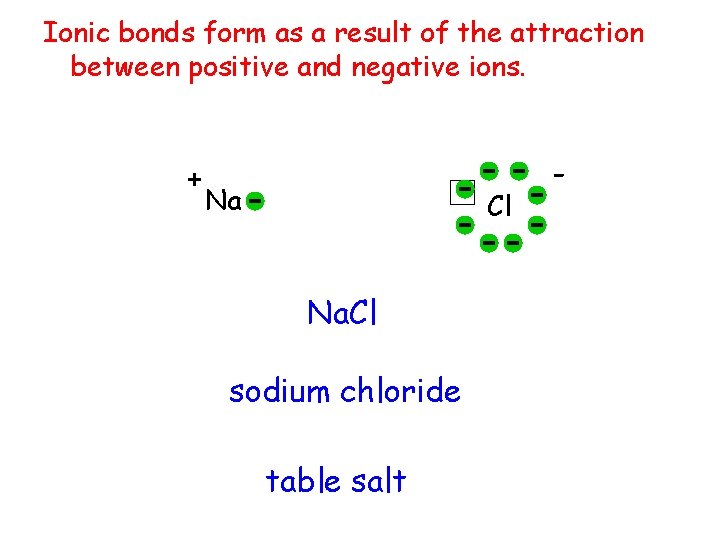
Ionic bonds form as a result of the attraction between positive and negative ions. + Na Cl Na. Cl sodium chloride table salt -

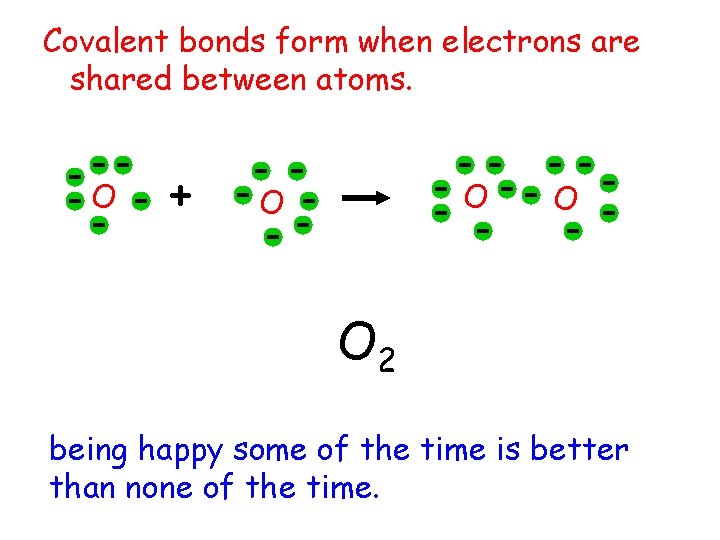
Covalent bonds form when electrons are shared between atoms. O + O O 2 being happy some of the time is better than none of the time.

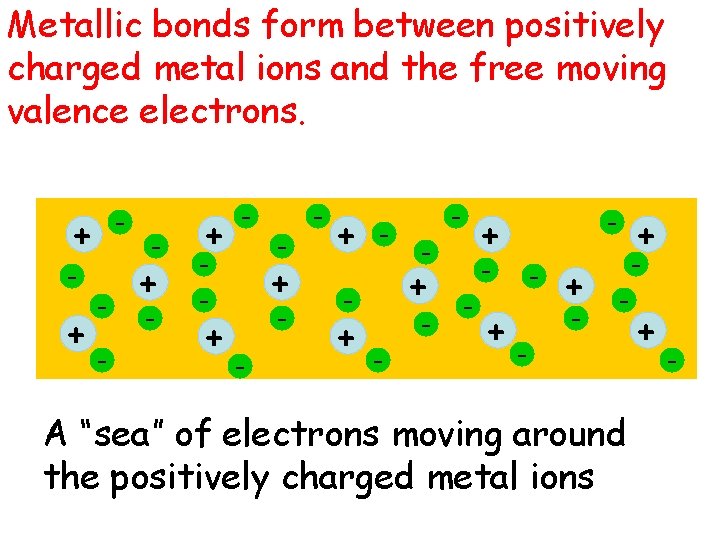
Metallic bonds form between positively charged metal ions and the free moving valence electrons. - + - + - - - + - + - - A “sea” of electrons moving around the positively charged metal ions + -