atomic emission spectrometry AES Atomic emission spectrometry Flame











- Slides: 27

atomic emission spectrometry (AES) Atomic emission spectrometry Flame: e. g. (i) flame atomic emission spectrometry (FAES) (ii) flame photometry Plasma: e. g. (iii) Inductively coupled plasma – atomic emission spectrometry (ICP-AES) or Inductively coupled plasma – optical emission spectrometry (ICP-OES)

Atomic Emission Spectrometry In atomic emission spectrometry (AES) there is no need for a radiation source as in (AAS) because atoms are thermally excited (e. g. by thermal energy of a flame or in a plasma) *AES is based on atomic excitations e. g. in: flame, plasma,

1 - Flames (relatively low temperature) - (i) Flame atomic emission spectrometry (FAES): A monochromator (a grating, mirrors) is used to select the wavelength used for measurements. - (ii) Flame photometry: Optical filters are used to choose the wave length for measurements.

Main principle of (FAES) Excitation of the atoms is achieved by thermal energy of the flame. When the excited atoms relax to the ground state, they emit characteristic radiations. These characteristic radiations allow the qualitative determination of the analyte and the intensity of emission allows for the quantitative estimation of the analyte. A calibration curve (using a number of standard solutions) is used to determine the analyte concentration.

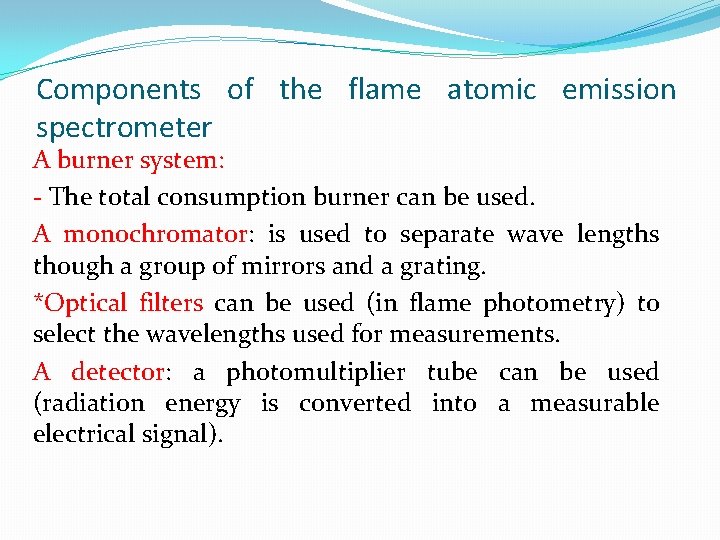
Components of the flame atomic emission spectrometer A burner system: - The total consumption burner can be used. A monochromator: is used to separate wave lengths though a group of mirrors and a grating. *Optical filters can be used (in flame photometry) to select the wavelengths used for measurements. A detector: a photomultiplier tube can be used (radiation energy is converted into a measurable electrical signal).

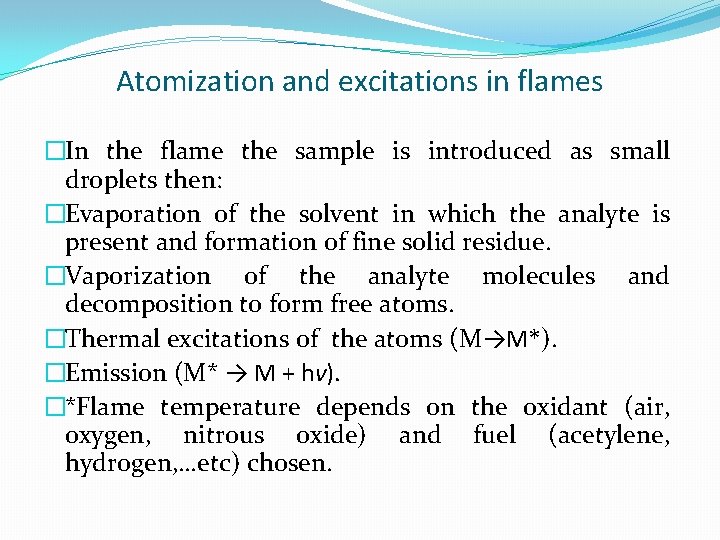
Atomization and excitations in flames �In the flame the sample is introduced as small droplets then: �Evaporation of the solvent in which the analyte is present and formation of fine solid residue. �Vaporization of the analyte molecules and decomposition to form free atoms. �Thermal excitations of the atoms (M→M*). �Emission (M* → M + hν). �*Flame temperature depends on the oxidant (air, oxygen, nitrous oxide) and fuel (acetylene, hydrogen, …etc) chosen.

Interferences �When other species in the sample emit the same emission line used for analysis, another line can be used for analysis.

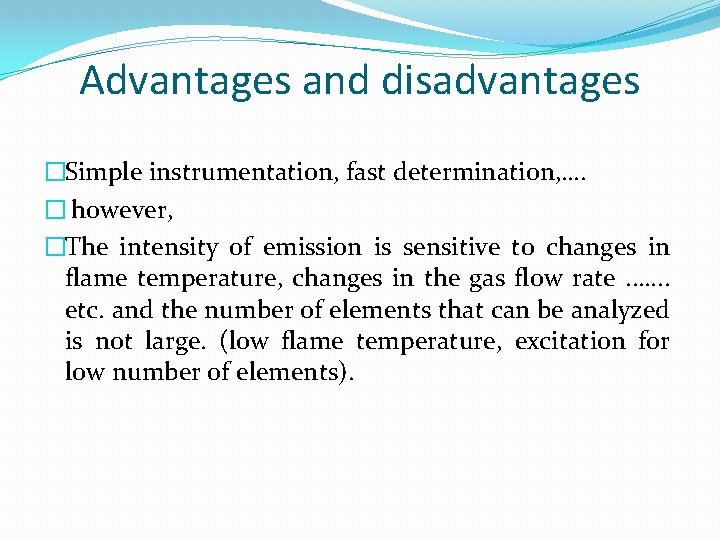
Advantages and disadvantages �Simple instrumentation, fast determination, …. � however, �The intensity of emission is sensitive to changes in flame temperature, changes in the gas flow rate. . …. . etc. and the number of elements that can be analyzed is not large. (low flame temperature, excitation for low number of elements).

Applications �The determination of some elements like the Cu, Na, K, Rb, Ca, Sr, Ba, … in biological and environmental samples.

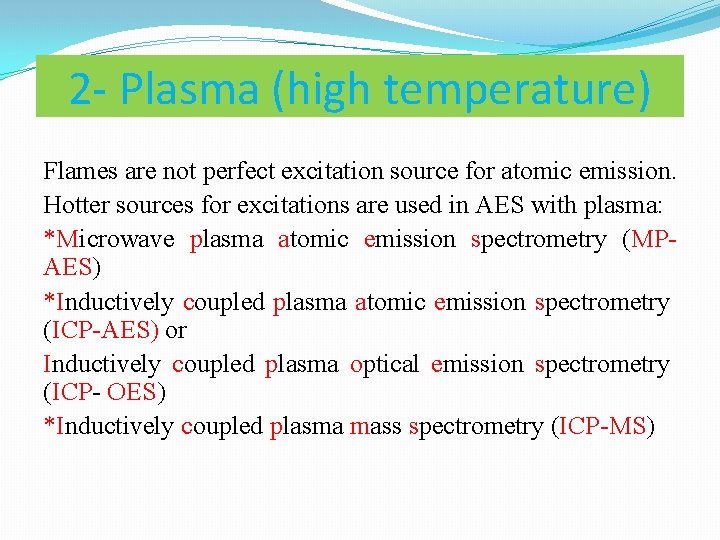
2 - Plasma (high temperature) Flames are not perfect excitation source for atomic emission. Hotter sources for excitations are used in AES with plasma: *Microwave plasma atomic emission spectrometry (MPAES) *Inductively coupled plasma atomic emission spectrometry (ICP-AES) or Inductively coupled plasma optical emission spectrometry (ICP- OES) *Inductively coupled plasma mass spectrometry (ICP-MS)

ICP �Introduction: � ICP-OES is based on the production and detection of line spectra of atoms emitted when the excited atoms relax to the ground state. �Because line spectra are specific for the element atom, qualitatively analysis can be carried out. Intensities of line spectra can be used for quantification. �Compared with other sources, plasma has: greater stability, higher temperature, higher ability to excite many elements altogether and higher sensitively. � Plasmas are much hotter than traditional flames and furnaces, they can, more efficiently, break down samples and excite (or ionize) the free atoms.

Principles of Operation • An argon inductively coupled plasma (up to 10, 000 K - hotter than microwave plasma) is used to remove solvent, atomize, and excite the analyte atoms (liquid samples) which has been nebulized in it. • Light intensity (emitted light from excited atoms) is measured using optical detection at the wavelengths characteristic of the elements to be determined. • ICP-OES is capable of measuring atomic and ionic emission (therefore, more wavelengths can be monitored). • These measurements can be compared with a standard to quantify the concentration of the elements in the analyte sample (calibration curve)

ICP �Uses very high temperature (provided by argon plasma) for atomization and excitation. �Relatively more atoms are formed. �Less chemical interferences. �Quantitative determination for a larger number of elements (compared to FAES). �The plasma is obtained using high voltage discharge through flowing argon. �The plasma is sustained by induction heating (the field of a radiofrequency coil). �A monochromator is used for selection of the wavelength used for analysis. �A photomultiplier tube can be used as a detector (extremely sensitive light detector (light of suitable energy). �Dynodes serve as an electron multiplier.

Plasma �Plasma: a homogeneous mixture of atoms, electrons and ions at very high temperature.

�Inductively coupled plasma: �High voltage discharge through flowing argon creates electrons which initiate the plasma. �The plasma temperature reaches 10000°C. �The plasma is held in place by a magnetic field, in the form of a fire ball. � The sample droplets or aerosol enters the fire ball where the atoms and ions of the analyte are formed, excited. �As the excited atoms relax to the ground state they emit characteristic lines.

�(torch plasma): �Three concentric quartzes tubes, having the same axis. �The larger one is about 2. 5 cm, on the top there is an induction coil (radio frequency induction coil). �Flow of current in the coil creates a magnetic field. �Argon gas flows through the tubes (about 15 l min-1) �The high temperature due to the glow of argon (10000°C) make it necessary to cool the surface of the quartz tube. �A tangential argon is used to cool the surface of the quartz tube.

�Large droplets are excluded and the finest analyte droplets (aerosol) are swept in argon for analysis. �In the analysis region, the temperature is about 6000°C. �The emitted radiation from the torch is directed to the monochromator and detected by a photomultiplier tube. �results are presented as intensity vs. wavelength (λ). �About 70 elements can be measured quickly, a large number from them can be measured at concentration of below ppb.

�Interferences: �In ICP because of the formation of many spectral lines, there is a high possibility for spectral interference: �Solution: �It is possible to measure at another resonance line for the element. �In ICP, there is little or no chemical interference (high temperature, no oxygen, argon environment). �In ICP there is no ionization interference (ionization of argon leads to the formation of large amount of electrons) (Le Chatelier principle)

�Advantages: Quick determination, little interferences compared to AAS, large droplets are excluded (compared to FAES)…………. . �however: �large amount of argon is required. �energy required is relatively high.

�Analytes (solution) are delivered to the nebulizer by a pump. �The nebulizer transforms the analyte solution into an aerosol (with the aid of argon) in the spray chamber.

�ultrasonic nebulizers: �Analyte samples are pumped on the surface of a piezoelectric crystal (vibrating in high frequency). Samples are converted into homogeneous aerosols that can be driven into the atomizer. �Ultrasonic is better than pneumatic nebulization (finer and more homogeneous droplets) �pneumatic nebulizers. �liquid sample is transformed into a fine spray (a gas is used)

Selection of the wavelength of measurement �A monochromator or polychromator (multiple exit slits and different λ values) is used to separate polychromatic radiation into individual wavelengths thus, emission can be identified and quantified. �Multielement analysis (a polychromator can measure them all together). �To minimize interferences, a number of characteristic lines are used for each element. �The light emitted by the atoms of an element in the ICP is converted to an electrical signal that can be measured quantitatively.

Detector �A photomultiplier tube. �A charge-coupled device (CCD) detector (a highly sensitive photon detector).

Calibration • • The intensity of the signal is compared to previously measured signals intensities of known concentrations of the element. *Each element has many specific wavelengths in the spectrum that could be used for analysis.

Advantages • No radiation source is required • Safe (no flammable gases) • Simultaneous multi-element analysis (qualitative and quantitative, sub-ppb level) (up to 73 elements) • Wide dynamic range • However, Not as sensitive as ICP-MS No isotope determination (compared to ICP-MS).

Applications Ø Most of the elements of the periodic table can be determined by ICP-OES with low limits of detection. Quantitative analysis of elements in: Ø - water Ø - soil Ø - sediment Ø - environmental samples. Ø

Inductively coupled plasma mass spectrometry (ICP-MS) �ICP-MS is capable of detecting elements at very low concentration. �ICP-MS combines an ICP with a mass spectrometer. �Atomization and ionization occurs in the ICP. �Ions are separated (according to their mass/charge (m/z) ratio), detected by the mass spectrometer and presented as (m/z) vs. signal intensity. �In the mass spectrum, the signal intensity is proportional to analyte concentration in the sample. �Advantages: �Very high sensitivity. �Isotopes determination.