ATOC 4720 class 12 n 1 n n

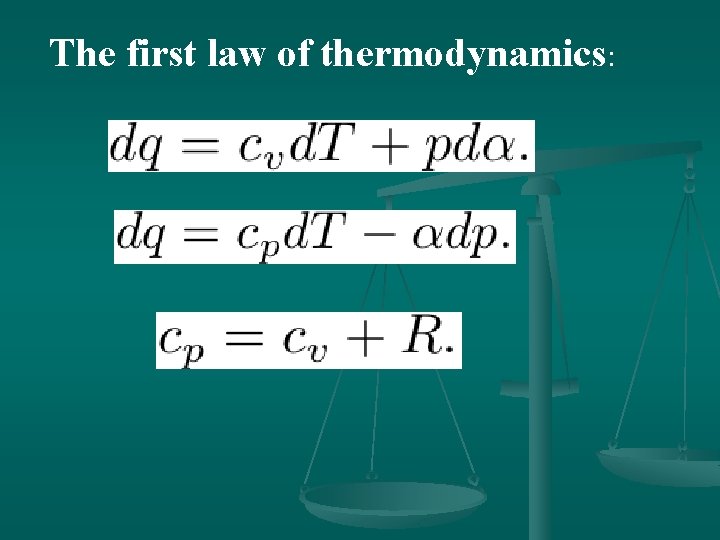






- Slides: 18

ATOC 4720: class 12 n 1. n n Enthalpy 2. Latent heat 3. Adiabatic processes
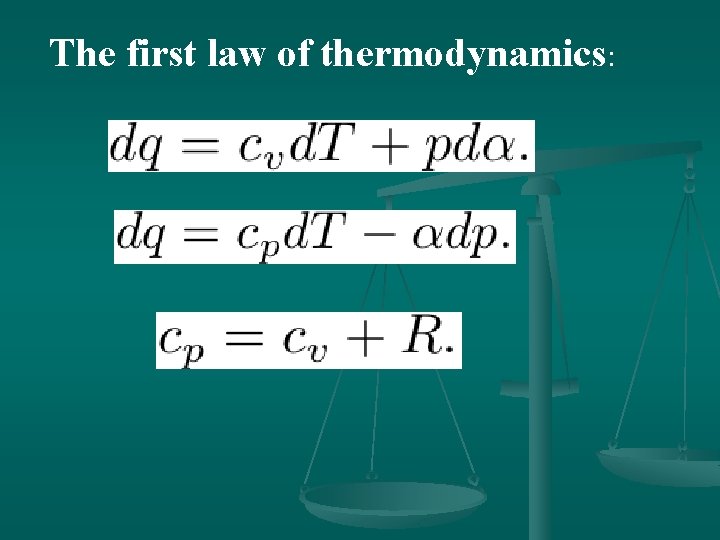
The first law of thermodynamics:

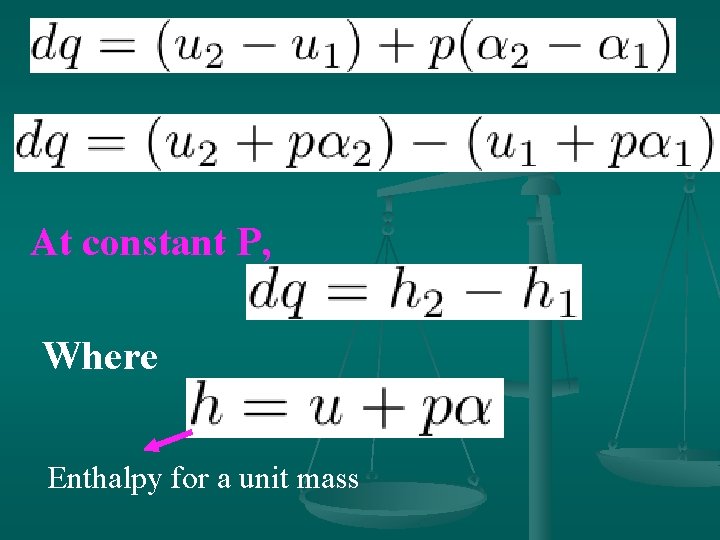
4. Enthalpy If heat is added to a material at constant pressure, so that the specific volume of the material increases from a 1 to a 2, the work done by a unit mass of the material is p(a 2 -a 1). Therefore, the heat dq added to a unit mass of the material at constant pressure is given by

At constant P, Where Enthalpy for a unit mass

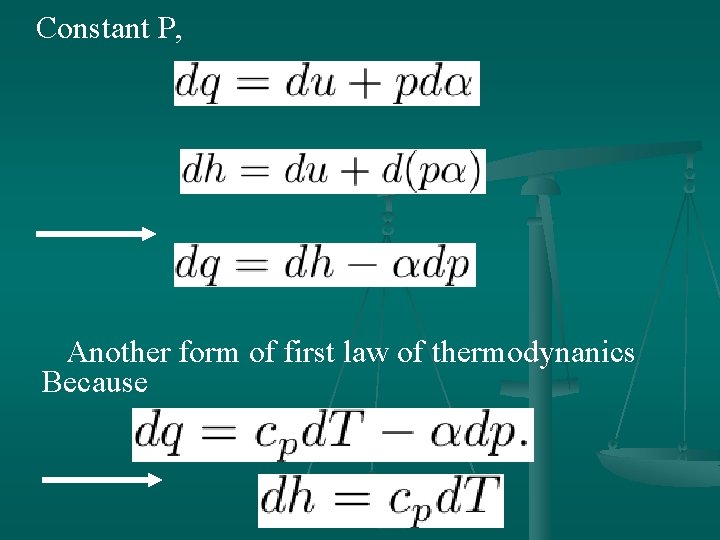
Constant P, Another form of first law of thermodynanics Because

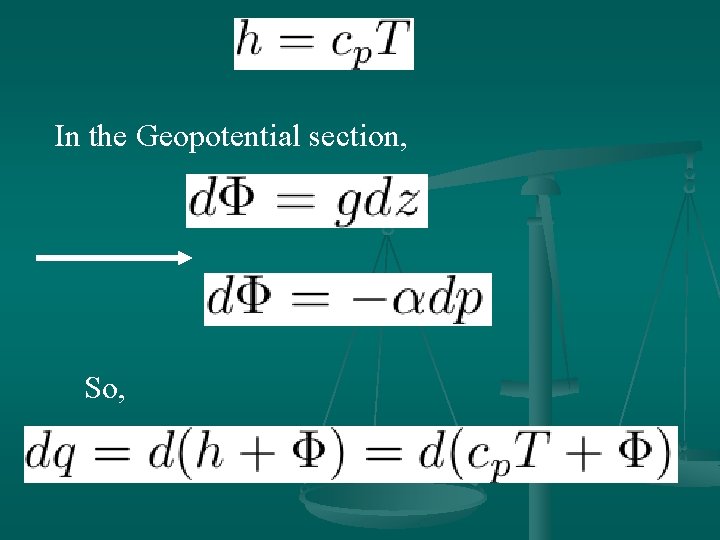
In the Geopotential section, So,

2. Latent heats Under certain conditions, heat may be supplied to a substance without changing its temperature. Ice -- water -- vapor; The latent heat of melting is the heat required to convert a unit mass of a material from the solid to liquid phase without a change in temperatuer.

The temperature at which this phase change occurs is called the melting point. At normal atmospheric pressure and T, the latent heat of melting of water substance is (fusion) Latent heat of vaporization is the heat required To convert a unit mass of material liquid--vapor phase without change of T. Normal P and 0 C, (condensation)

Melting & boiling points--P; Latent heat of fusion and vaporization-T;

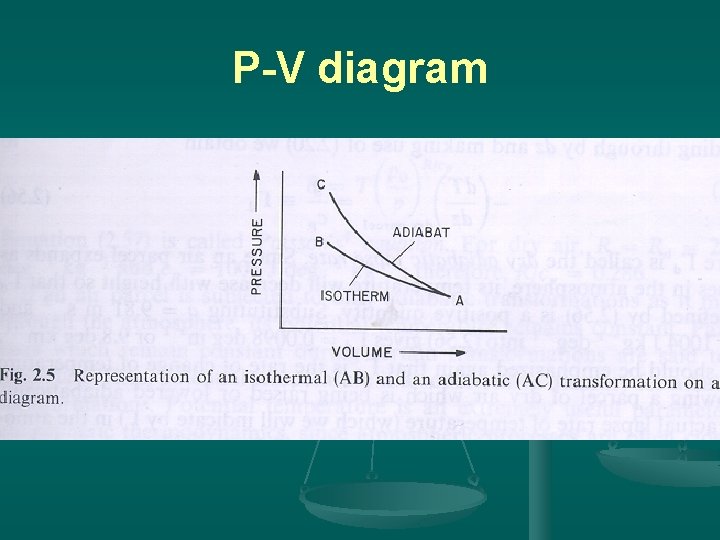
3. Adiabatic processes Concept: If a material changes its physical state (P, T, V) without gaining or losing heat--adiabatic. A good tool to represent the process: p-V diagram.

P-V diagram

An air parcel Mixing in the atm can be accomplished by two processes: Below 100 km; Well-defined air parcels above 100 km.

An air parcel: n n n Thermally insulated: adiabatic (rises or sink); Always at the environmental P; (env. Air assumed to be in hydrostatic equilibrium; Moving slowly enough that its kinetic energy is a negligible fraction of its total energy; [Real air do not satisfy all; but important to understand physical processes: z mix. & motion]

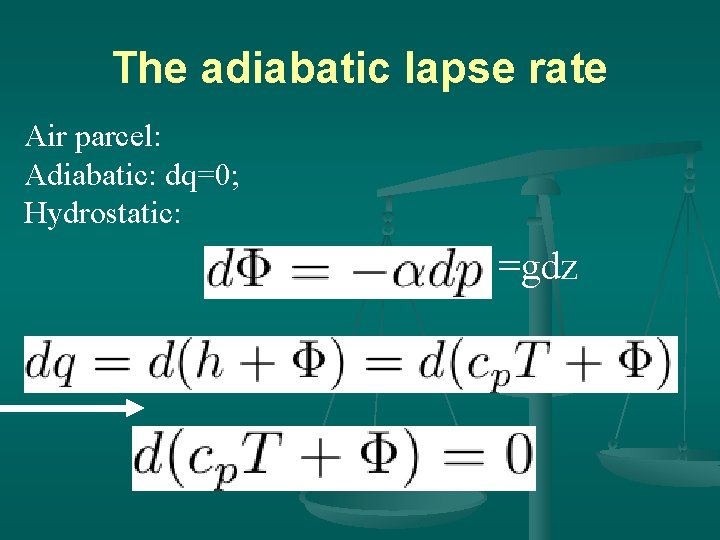
The adiabatic lapse rate Air parcel: Adiabatic: dq=0; Hydrostatic: =gdz

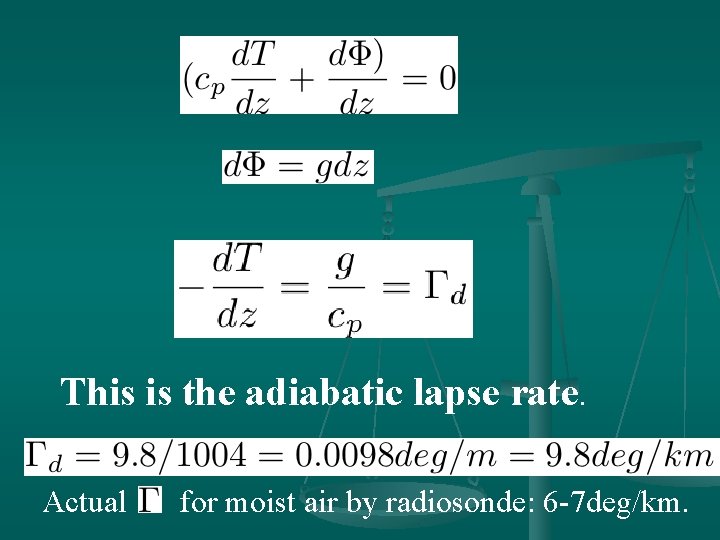
This is the adiabatic lapse rate. Actual for moist air by radiosonde: 6 -7 deg/km.

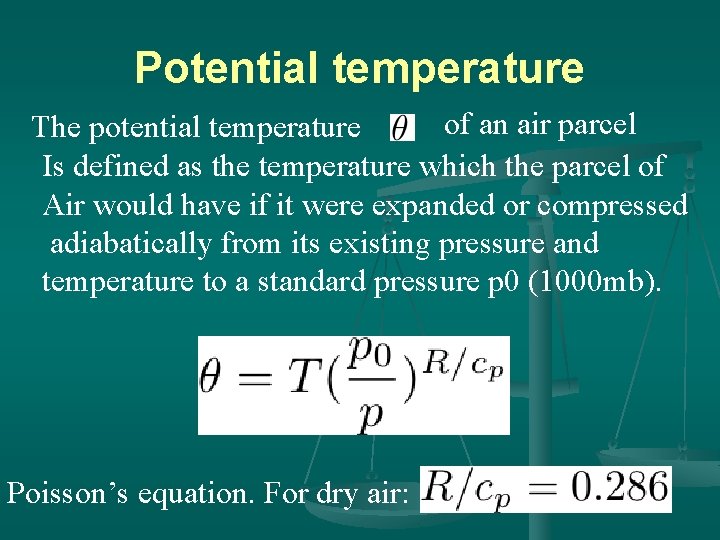
Potential temperature of an air parcel The potential temperature Is defined as the temperature which the parcel of Air would have if it were expanded or compressed adiabatically from its existing pressure and temperature to a standard pressure p 0 (1000 mb). Poisson’s equation. For dry air:

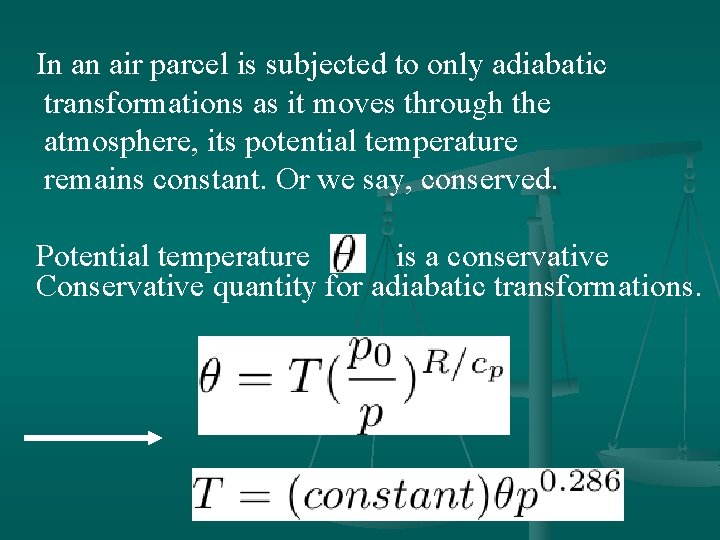
In an air parcel is subjected to only adiabatic transformations as it moves through the atmosphere, its potential temperature remains constant. Or we say, conserved. Potential temperature is a conservative Conservative quantity for adiabatic transformations.

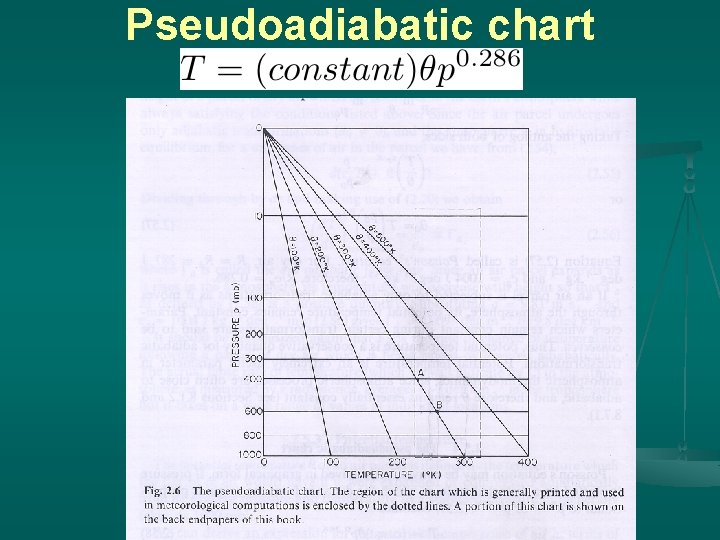
Pseudoadiabatic chart