ASEAN Guidelines on GMP for Traditional Medicines Health












- Slides: 47

ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements (TM/HS) EVALUATION OF CORRECTIVE ACTION AND PREVENTIVE ACTION (CAPA) Prepared by: Malaysia Approved by: ASEAN TMHS GMP Task Force Endorsed by: ASEAN TMHS Product Working Group

OUTLINE • • • What is CAPA Basic CAPA System Root Cause Analysis and the Tools CAPA Regulatory challenges and common issues Summary ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA 2

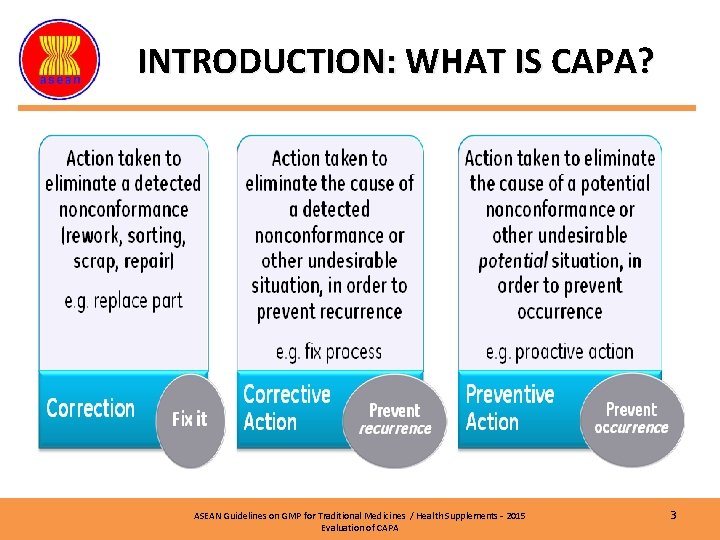
INTRODUCTION: WHAT IS CAPA? ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA 3

WHAT IS CAPA? (2) CORRECTIVE ACTION ØEliminate detected nonconformity PREVENTIVE ACTION Ø Prevent nonconformity occurrence ü The HEART of an effective quality management system ü CAPA methodology is for continuous improvement of product & process ü To enhance product & process understanding and eliminate potential non-conformance. As per ICH Q 10; Pharmaceutical Quality System ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA 4

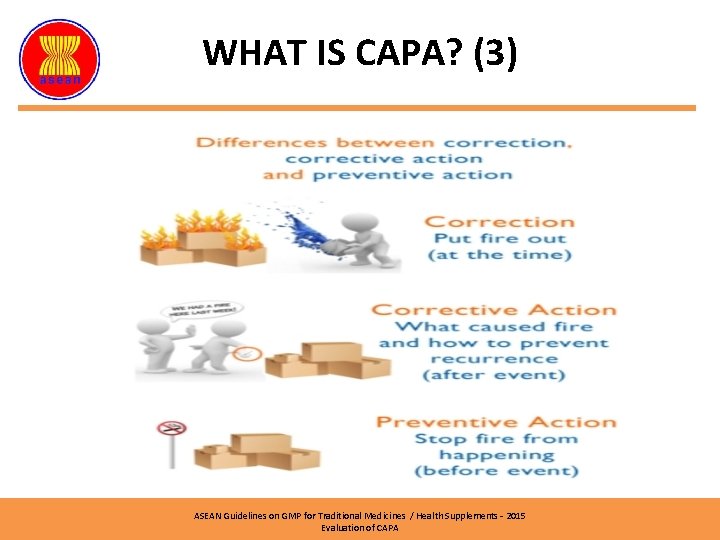
WHAT IS CAPA? (3) ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

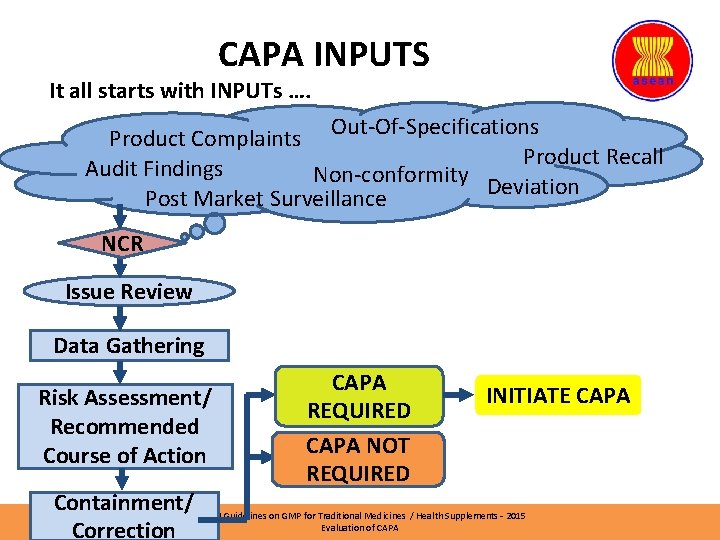
CAPA INPUTS It all starts with INPUTs …. Out-Of-Specifications Product Complaints Product Recall Audit Findings Non-conformity Deviation Post Market Surveillance NCR Issue Review Data Gathering Risk Assessment/ Recommended Course of Action CAPA REQUIRED CAPA NOT REQUIRED INITIATE CAPA Containment/ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA Correction

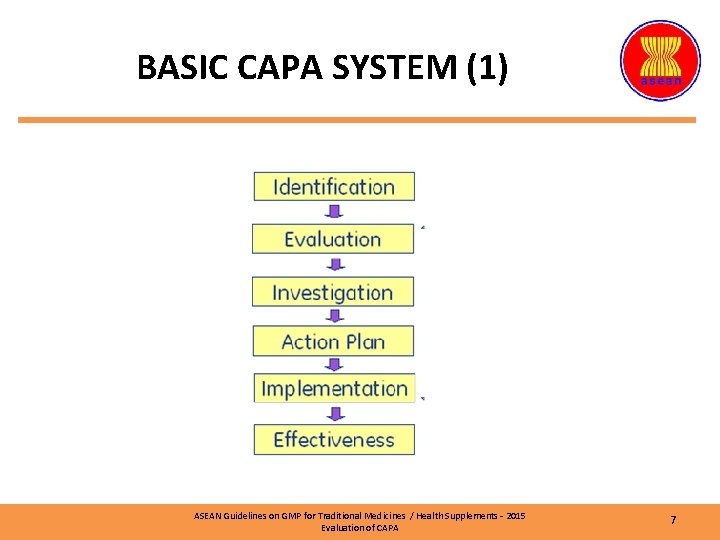
BASIC CAPA SYSTEM (1) ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA 7

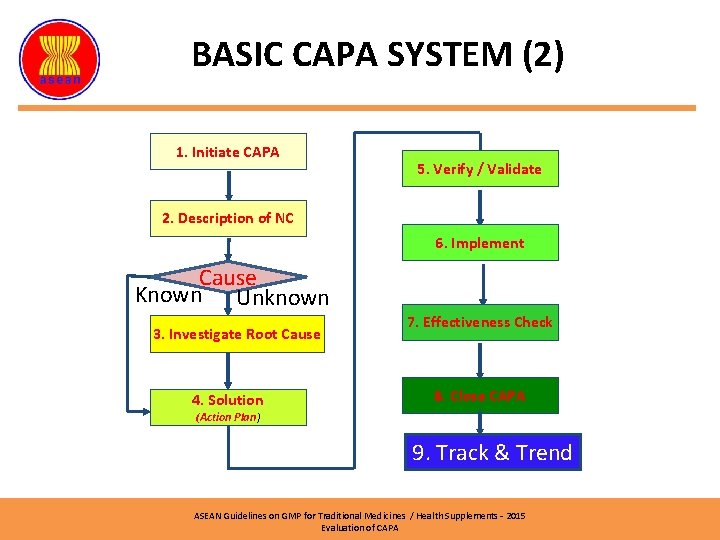
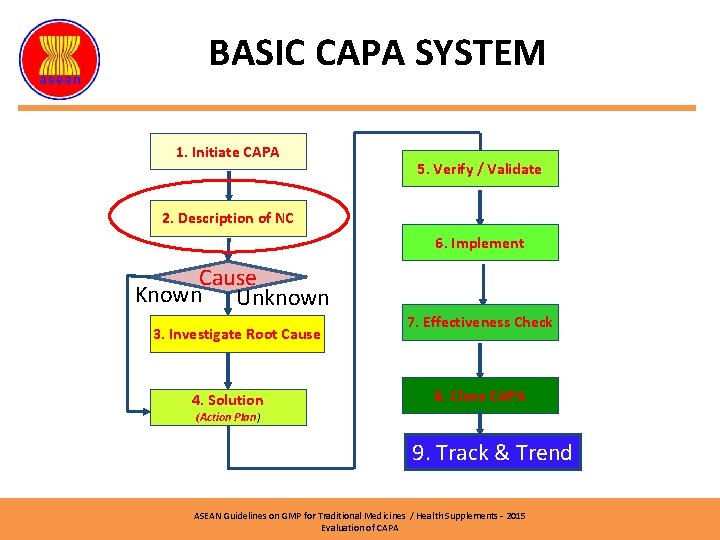
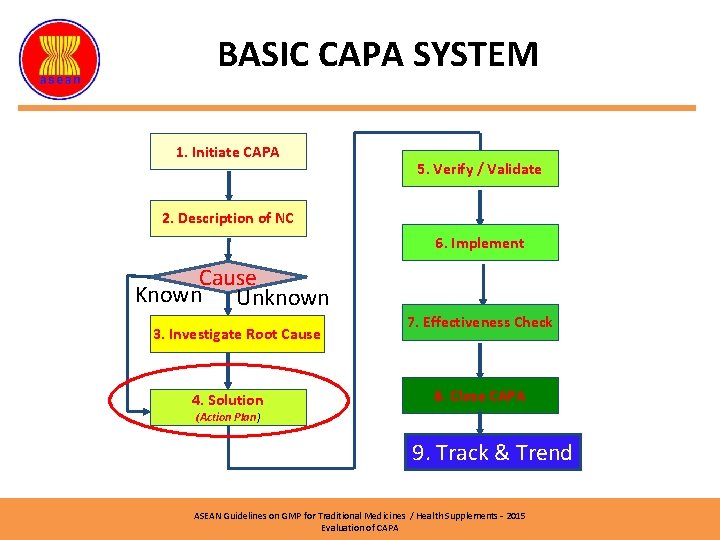
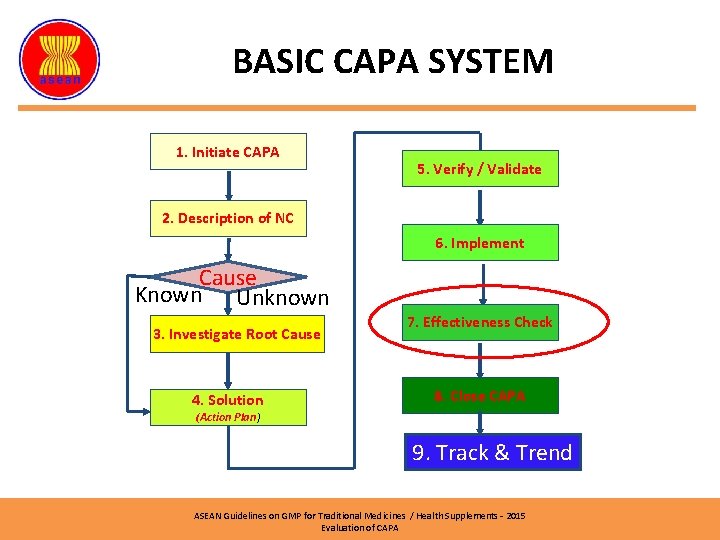
BASIC CAPA SYSTEM (2) 1. Initiate CAPA 5. Verify / Validate 2. Description of NC 6. Implement Cause Known Unknown 3. Investigate Root Cause 4. Solution 7. Effectiveness Check 8. Close CAPA (Action Plan) 9. Track & Trend ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

BASIC CAPA SYSTEM 1. Initiate CAPA 5. Verify / Validate 2. Description of NC 6. Implement Cause Known Unknown 3. Investigate Root Cause 4. Solution 7. Effectiveness Check 8. Close CAPA (Action Plan) 9. Track & Trend ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

DESCRIPTION OF NONCONFORMANCE • Describe the non-conformance precisely – What, when, where, who, why & how. ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

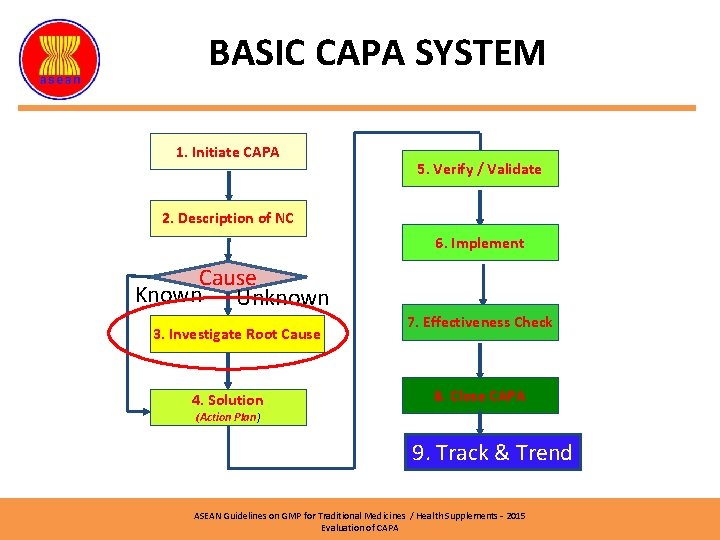
BASIC CAPA SYSTEM 1. Initiate CAPA 5. Verify / Validate 2. Description of NC 6. Implement Cause Known Unknown 3. Investigate Root Cause 4. Solution 7. Effectiveness Check 8. Close CAPA (Action Plan) 9. Track & Trend ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

INVESTIGATION CHECKLIST • Describe the fast fix that was taken, Is it working? • Identify owner and those involved. • Gather information: – – – Data Employee Input Flowcharts of the process Procedures Records – (quantitative data) • Has the problem occurred in the past? • Identify Root Cause. ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

RULES OF INVESTIGATION • Use proven root cause analysis tools • Think “out – of – the – box” • Take the time needed • Put a plan together ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

WHAT MAKES A GOOD ROOT CAUSE? • Document the primary cause (s) of the nonconformance – Understand the problem statement – Don’t assume that people know or understand the problem well enough to determine root cause – Data collection and analysis • Involve people who have an understanding of the problem • Used Cause and Effect principle • Facilitate the Root Cause Analysis – Provide the knowledge of RCA tools • When solved, the problem will not recur ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

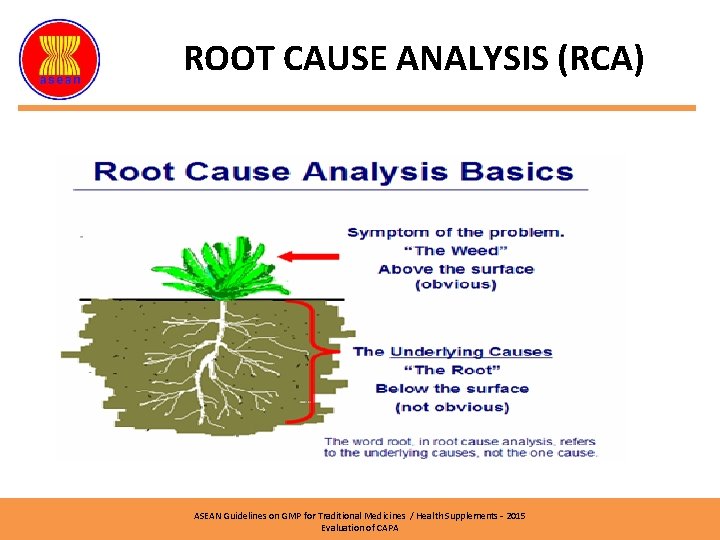
ROOT CAUSE ANALYSIS (RCA) ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

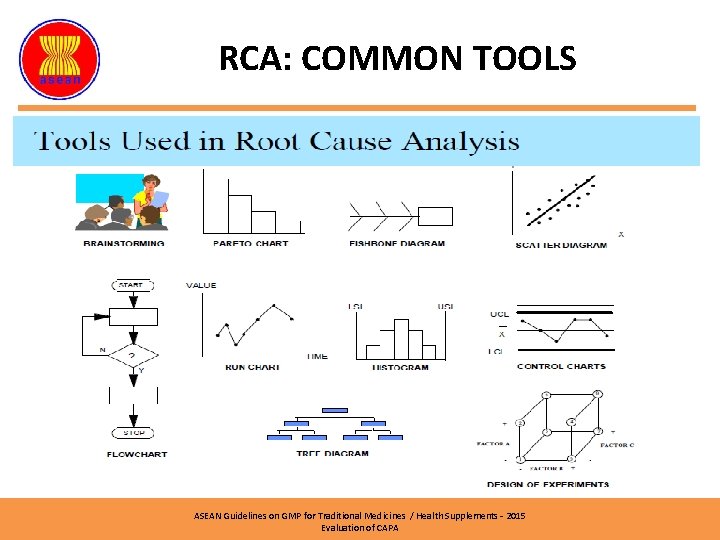
RCA: COMMON TOOLS ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

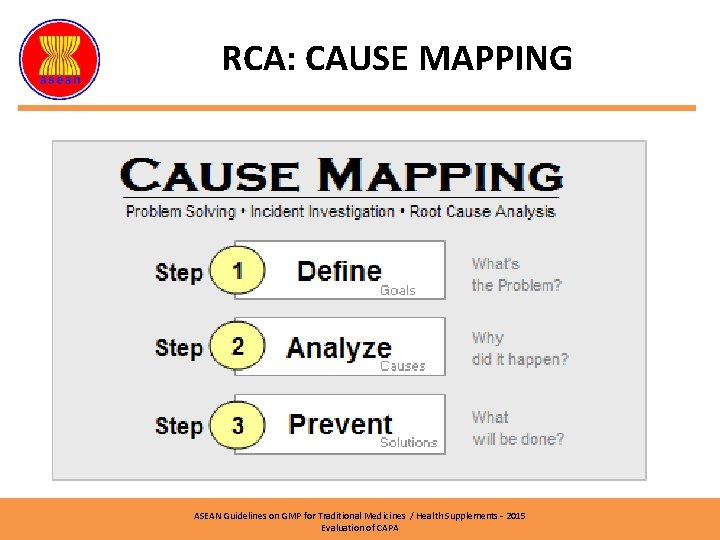
RCA: CAUSE MAPPING ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

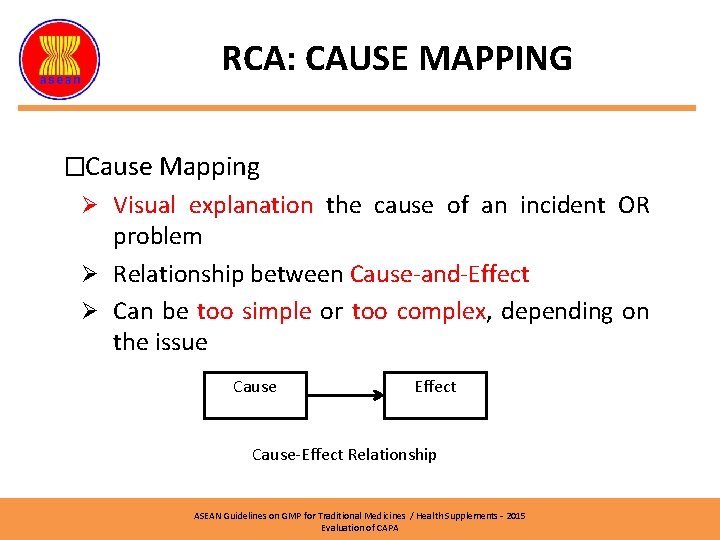
RCA: CAUSE MAPPING �Cause Mapping Ø Visual explanation the cause of an incident OR problem Ø Relationship between Cause-and-Effect Ø Can be too simple or too complex, depending on the issue Cause Effect Cause-Effect Relationship ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

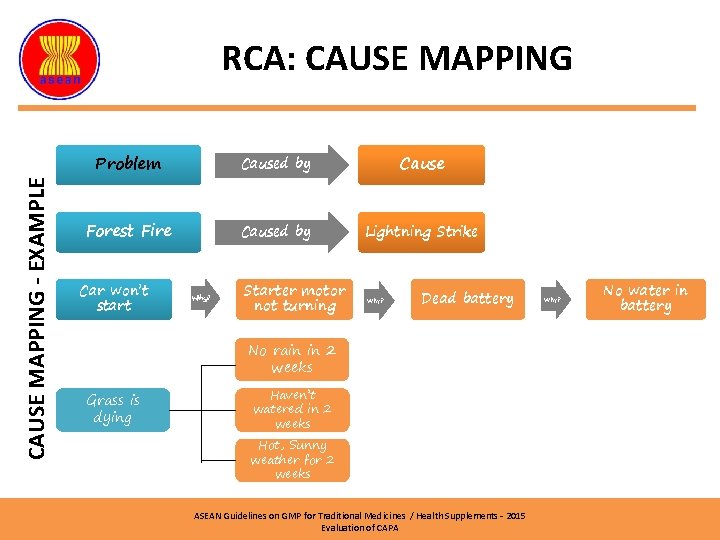
CAUSE MAPPING - EXAMPLE RCA: CAUSE MAPPING Problem Caused by Cause Forest Fire Caused by Lightning Strike Car won’t start Why? Starter motor not turning Why? Dead battery No rain in 2 weeks Grass is dying Haven’t watered in 2 weeks Hot, Sunny weather for 2 weeks ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA Why? No water in battery

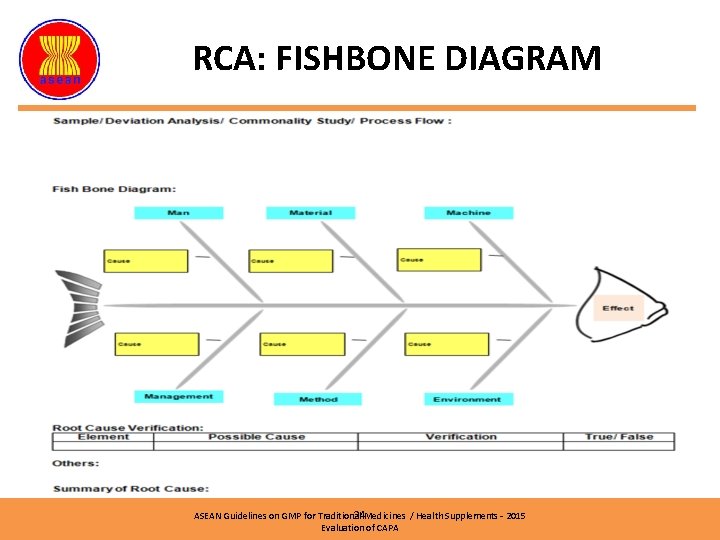
RCA: FISHBONE DIAGRAM Ishikawa/ Fishbone Diagram ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

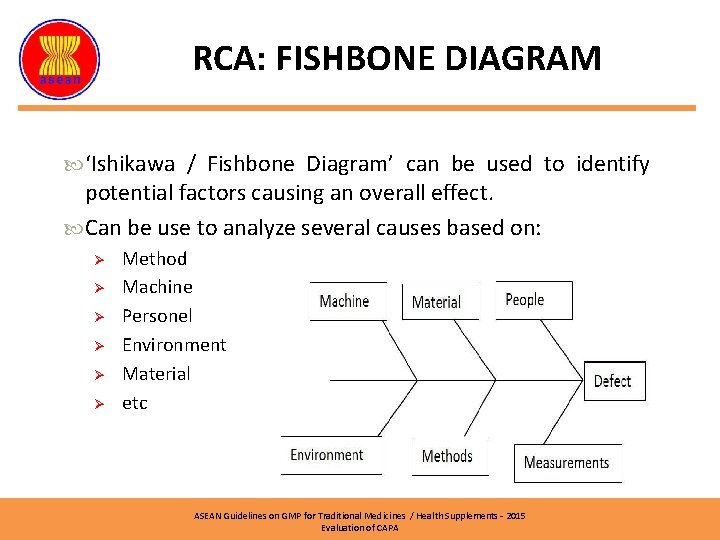
RCA: FISHBONE DIAGRAM ‘Ishikawa / Fishbone Diagram’ can be used to identify potential factors causing an overall effect. Can be use to analyze several causes based on: Ø Ø Ø Method Machine Personel Environment Material etc ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

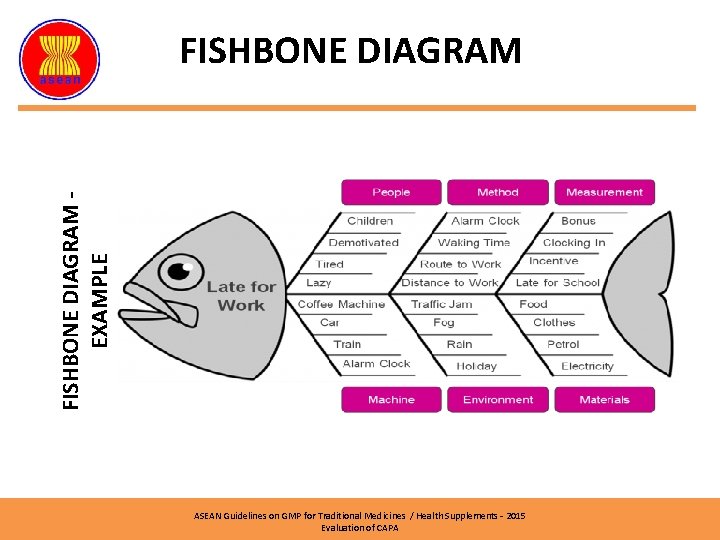
FISHBONE DIAGRAM EXAMPLE FISHBONE DIAGRAM ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

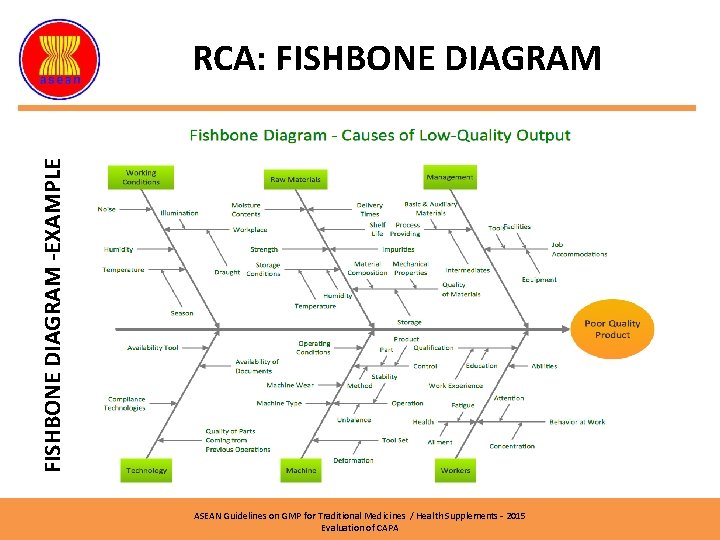
FISHBONE DIAGRAM -EXAMPLE RCA: FISHBONE DIAGRAM ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

RCA: FISHBONE DIAGRAM ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 24 Evaluation of CAPA

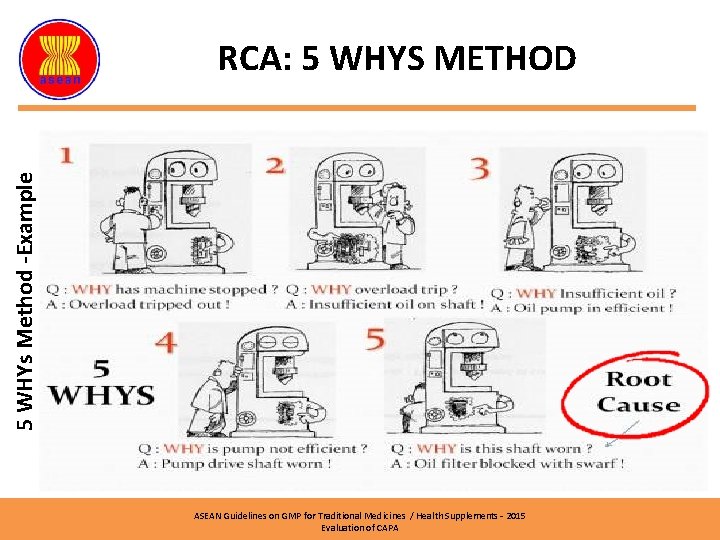
RCA: FISHBONE DIAGRAM (5 WHYs Method) Why, why, why Kaji Cegah ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

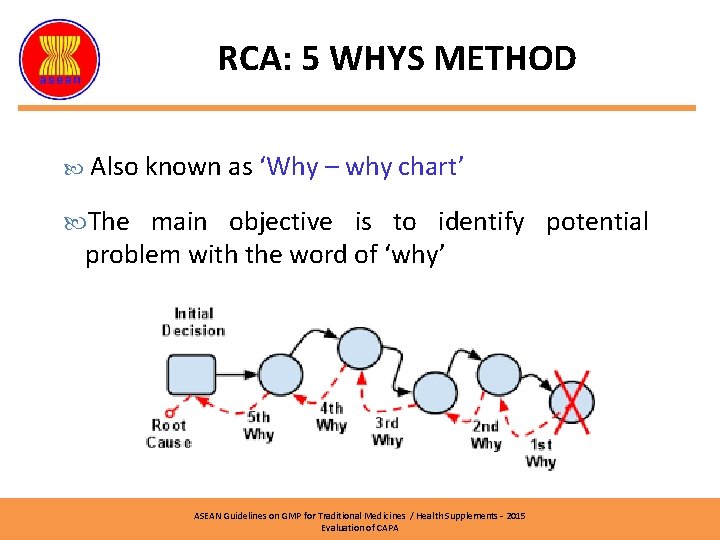
RCA: 5 WHYS METHOD Also known as ‘Why – why chart’ The main objective is to identify potential problem with the word of ‘why’ ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

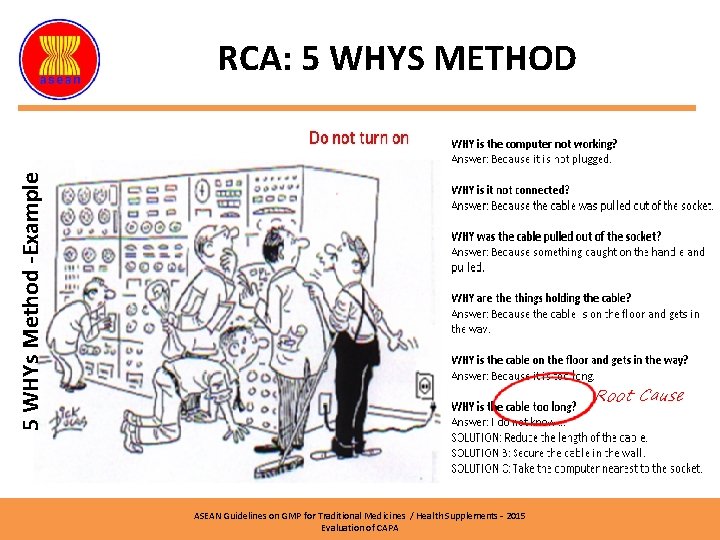
5 WHYs Method -Example RCA: 5 WHYS METHOD ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

5 WHYs Method -Example RCA: 5 WHYS METHOD Root Cause ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

5 WHYs Method -Example RCA: 5 WHYS METHOD ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

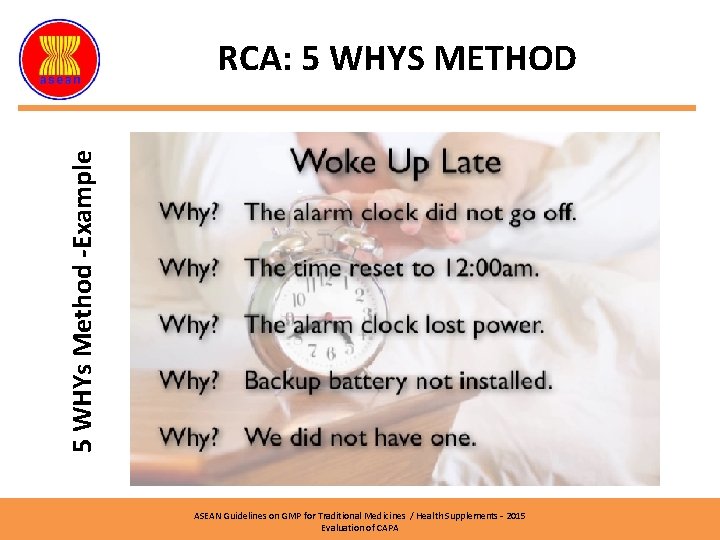
5 WHYs Method -Example RCA: 5 WHYS METHOD Finding: A cockroach was found under a mixer in Mixing Room 1 Why the cockroach died? Answer: Eating bait Why it ate bait? Answer: There was a bait in the Mixing Room 1 Why there was a bait in Mixing Room 1? Answer: Requirement of pest control procedure. Why the SOP required cockroach bait inside the production area? Answer: The personnel who prepared the SOP didn’t aware the GMP principle. Why he didn’t aware the GMP requirement? Answer: The personnel never been trained accordingly. Root Cause ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

BASIC CAPA SYSTEM 1. Initiate CAPA 5. Verify / Validate 2. Description of NC 6. Implement Cause Known Unknown 3. Investigate Root Cause 4. Solution 7. Effectiveness Check 8. Close CAPA (Action Plan) 9. Track & Trend ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

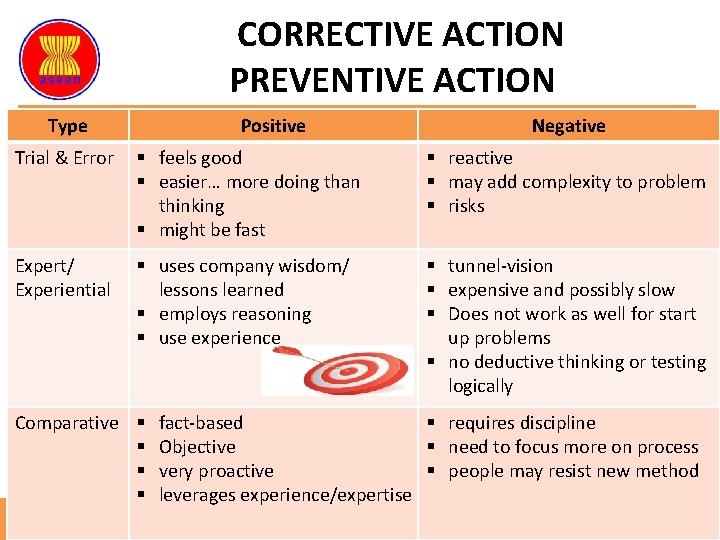
CORRECTIVE ACTION PREVENTIVE ACTION Type Positive Negative Trial & Error § feels good § easier… more doing than thinking § might be fast § reactive § may add complexity to problem § risks Expert/ Experiential § uses company wisdom/ lessons learned § employs reasoning § use experience § tunnel-vision § expensive and possibly slow § Does not work as well for start up problems § no deductive thinking or testing logically Comparative § § fact-based § requires discipline Objective § need to focus more on process very proactive § people may resist new method leverages experience/expertise ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

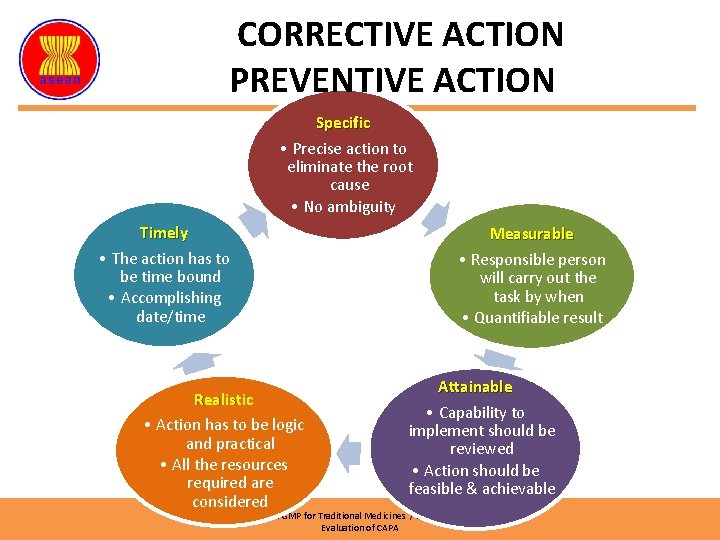
CORRECTIVE ACTION PREVENTIVE ACTION Specific • Precise action to eliminate the root cause • No ambiguity Timely • The action has to be time bound • Accomplishing date/time Realistic • Action has to be logic and practical • All the resources required are considered Measurable • Responsible person will carry out the task by when • Quantifiable result Attainable • Capability to implement should be reviewed • Action should be feasible & achievable ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

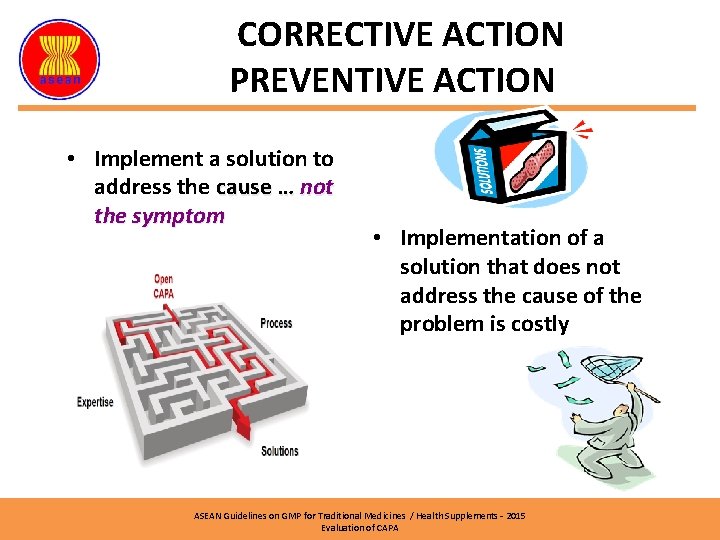
CORRECTIVE ACTION PREVENTIVE ACTION • Implement a solution to address the cause … not the symptom • Implementation of a solution that does not address the cause of the problem is costly ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

BASIC CAPA SYSTEM 1. Initiate CAPA 5. Verify / Validate 2. Description of NC 6. Implement Cause Known Unknown 3. Investigate Root Cause 4. Solution 7. Effectiveness Check 8. Close CAPA (Action Plan) 9. Track & Trend ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

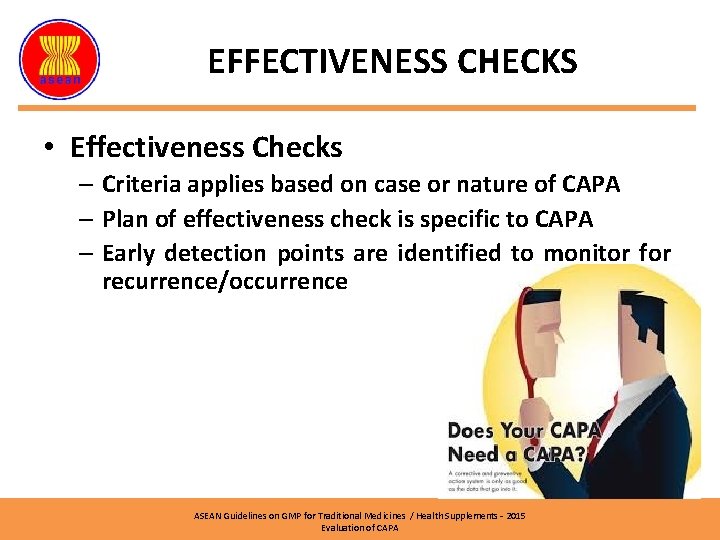
EFFECTIVENESS CHECKS • Effectiveness Checks – Criteria applies based on case or nature of CAPA – Plan of effectiveness check is specific to CAPA – Early detection points are identified to monitor for recurrence/occurrence ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

EFFECTIVENESS CHECKS • What to do when a effectiveness check fails, and what are the consequences? – Close the CAPA and open a new one? – Get an extension? – Leave the CAPA open and investigate why? ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

REGULATORY CHALLENGES • No submission of CAPA feedback • Feedback too simple or no cover letter e. g. One Page • Insufficient CAPA feedback e. g. : ‘We take note on your comment’ • No dateline/ timeframe • No CAPA • No supporting document ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

COMMON CAPA ISSUES • • • No procedure Did not understand the issue No commitment from top management No monitoring Did not identify RCA or wrong RCA ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

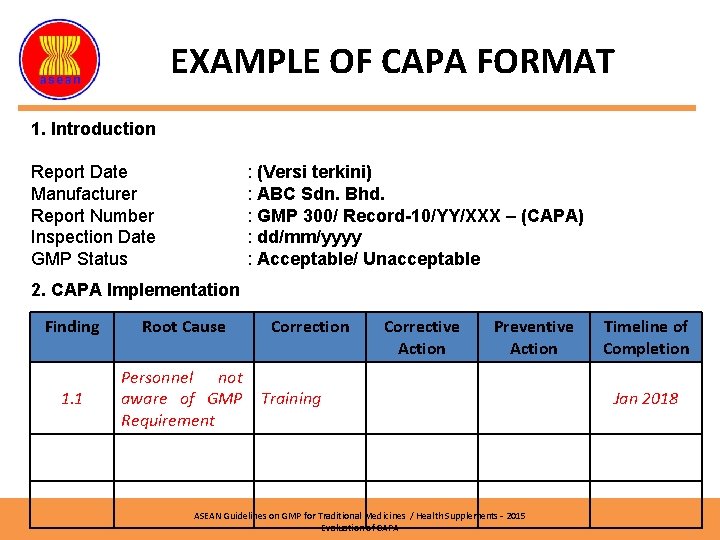
EXAMPLE OF CAPA FORMAT 1. Introduction Report Date Manufacturer Report Number Inspection Date GMP Status : (Versi terkini) : ABC Sdn. Bhd. : GMP 300/ Record-10/YY/XXX – (CAPA) : dd/mm/yyyy : Acceptable/ Unacceptable 2. CAPA Implementation Finding Root Cause 1. 1 Personnel not aware of GMP Requirement Correction Corrective Action Preventive Action Training ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA Timeline of Completion Jan 2018

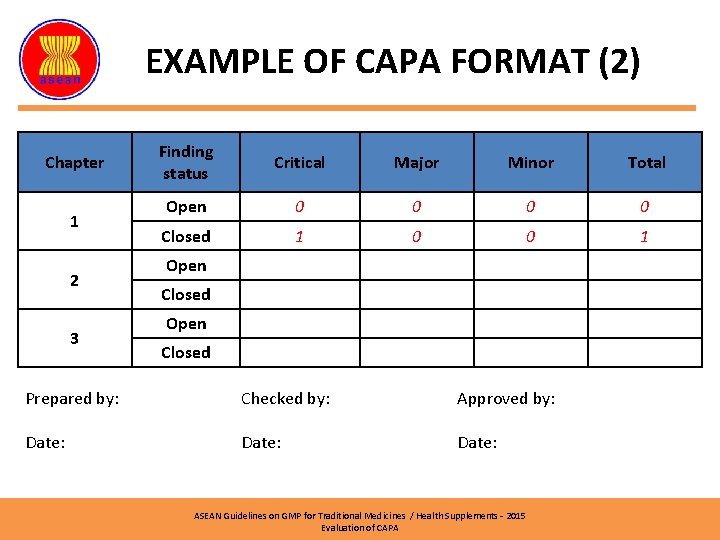
EXAMPLE OF CAPA FORMAT (2) Chapter 1 2 3 Prepared by: Date: Finding status Critical Major Minor Total Open 0 0 Closed 1 0 0 1 Open Closed Checked by: Approved by: Date: ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

CAPA CLOSURE • The CAPA implementation need to follow by the monitoring of its effectiveness • Various source of data need to be considered when analyze the CAPA • CAPA documentation need to be checked and verify accurately before being stored ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

CONTINUOUS IMPROVEMENT I can close the CAPA because have taken the action I’m confident that I’ve solved the problem and ensure it won’t be repeat again ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

CONFIRMATION OF CAPA The next inspection? ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

BENEFITS OF GOOD CAPA SYSTEM • • • Assurance that quality issues are resolved Customer complaints are reduced Promotion of continuous improvement An aligned method for problem solving Regulatory requirements are met Business needs are met ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

SUMMARY • A mature CAPA system can serve as a useful tool for analysing past events, correcting existing non conformities and preventing future events. • CAPA will be useless if there is no commitment from top management of company or just simply prepared to meet the regulatory requirement ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA

ASEAN Guidelines on GMP for Traditional Medicines / Health Supplements – 2015 Evaluation of CAPA 47
 Nafdac gmp guidelines
Nafdac gmp guidelines Gmp
Gmp Glencoe health chapter 19
Glencoe health chapter 19 Federal agency for medicines and health products
Federal agency for medicines and health products Veterinary medicines directorate
Veterinary medicines directorate George's marvellous medicine story
George's marvellous medicine story Refrigerant management program
Refrigerant management program Mobile dispensing method belong to
Mobile dispensing method belong to Niyog niyogan health benefits
Niyog niyogan health benefits European directorate for the quality of medicines
European directorate for the quality of medicines Which legislation helped solve trusts
Which legislation helped solve trusts Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Medicines learning portal
Medicines learning portal Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Ratiograstin
Ratiograstin Medicines complete
Medicines complete Cqc medicines management
Cqc medicines management Medicines information centre
Medicines information centre Medicines complete martindale
Medicines complete martindale Pharmaceutical schedule
Pharmaceutical schedule National medicines policy
National medicines policy Ggc medicines
Ggc medicines Seato and asean
Seato and asean 3 pillars of asean
3 pillars of asean The three pillars of asean
The three pillars of asean Asean culture
Asean culture Pt barra asean shipping
Pt barra asean shipping Rubrik penilaian membaca teks
Rubrik penilaian membaca teks Lambang asean berupa seikat batang padi yang menggambarkan
Lambang asean berupa seikat batang padi yang menggambarkan Peta konsep islam di asia tenggara
Peta konsep islam di asia tenggara Asean working group on environmentally sustainable cities
Asean working group on environmentally sustainable cities Asean quiz reviewer
Asean quiz reviewer Asean symbol meaning
Asean symbol meaning E-asean
E-asean Kepentingan malaysia menganggotai pertubuhan antarabangsa
Kepentingan malaysia menganggotai pertubuhan antarabangsa Keadaan alam vietnam
Keadaan alam vietnam Asean countries
Asean countries Asean women's entrepreneurship network
Asean women's entrepreneurship network Asean wildlife enforcement network
Asean wildlife enforcement network Asean socio-cultural community pillar
Asean socio-cultural community pillar Swot analysis thailand
Swot analysis thailand Awgcc
Awgcc Asean secretariat
Asean secretariat Asean countries
Asean countries Asean paises
Asean paises Yuyun simca
Yuyun simca Asean banking integration framework
Asean banking integration framework Asean graduate program
Asean graduate program