HARMONIZATION OF GMP INSPECTION IN THE EAST AFRICAN













- Slides: 16

HARMONIZATION OF GMP INSPECTION IN THE EAST AFRICAN COMMUNITY WHO Technical Briefing Seminar 5 th November 2014 Presented by: Kate Kikule (Mrs. ) Technical Officer (Rotational) World Health Organization Geneva, Switzerland 11/23/2020 1

Presentation Outline Facts about EAC Background to medicine regulation harmonization in EAC GMP TWG Achievements Challenges Plan for the future 11/23/2020 2

EAST AFRICAN COMMUNITY 11/23/2020 3

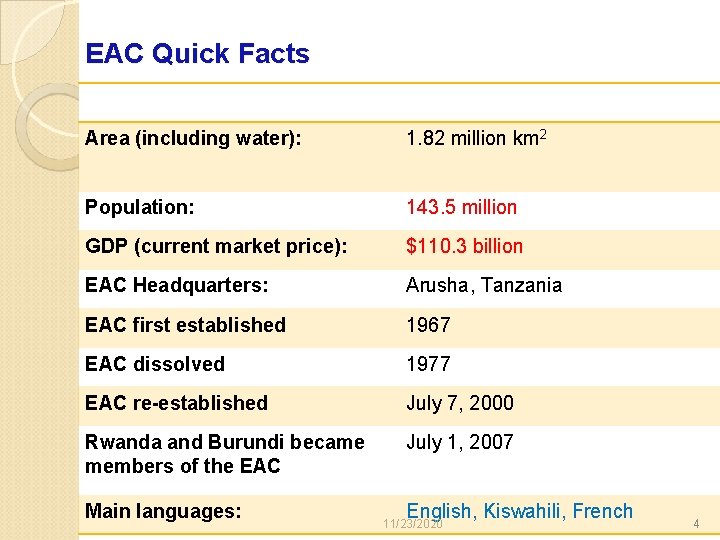
EAC Quick Facts Area (including water): 1. 82 million km 2 Population: 143. 5 million GDP (current market price): $110. 3 billion EAC Headquarters: Arusha, Tanzania EAC first established 1967 EAC dissolved 1977 EAC re-established July 7, 2000 Rwanda and Burundi became members of the EAC July 1, 2007 Main languages: English, Kiswahili, French 11/23/2020 4

Background to Harmonization in the EAC Year Activity Outcome 1999 EAC Treaty Cooperation among EAC States 2000 Directive of the EAC Council of Ministers Research, Policy and Health Systems Working group tasked to draft common Drug Policy and Harmonized drug regulation procedures 2001 Meeting of Technical staff from NMRAs in EAC Developed guidelines and application forms for registration of Veterinary Drugs 2005 EAC Customs Union Common external tariffs on medicines 2005 African Drug Regulators Conference, Recommendation to promote Addis Ababa harmonization using existing RECs 2005 Kampala meeting 2006 Nairobi Action plan for harmonization in the EAC 11/23/2020 Formation of TWGs 5

Background II Setback to the 2006 -2008 Action plan ◦ Slow progress of implementation ◦ Inadequate funding of TWGs However, the consortium of WHO, NEPAD, DFID and GTZ, expressed their interest in supporting RECs, (Johannesburg, Feb 2009) and called for funding proposals from RECs 11/23/2020 6

EAC Medicine Regulation harmonization project Funded by Bill and Melinda Gates Foundation Duration – 5 years Four(4) regional technical working groups were established ◦ Medicine registration – Tanzania ◦ Good Manufacturing Practice Inspection – Uganda ◦ Information Management Systems – Rwanda ◦ Quality Management Systems - Kenya 11/23/2020 7

EAC GMP TWG Composition The technical working group is composed of twelve members drawn from the six NMRAs of the Republics of Burundi, Kenya, Rwanda , Uganda and The United Republic of Tanzania (Mainland) and Tanzania (Zanzibar) 11/23/2020 8

Aim of the harmonization initiative Harmonized legal framework for GMP, guidelines and procedures for GMP Inspection Development of medicine regulatory curricula and training materials, including e-learning Improved regulatory systems for faster access of quality medicines on to the EAC market 11/23/2020 9

Achievements: Guidance Documents developed and approved for use within the EAC Manual for Good Manufacturing Practice Inspection Guidelines for: § GMP of medicinal products and related annexes § preparation of site master file for pharmaceutical manufacturing facilities § Training and Qualifications of GMP Inspectors Standard Operating Procedures for: § planning for GMP inspections § preparing for a GMP inspection § conducting a GMP inspection § preparing and reviewing a good manufacturing practice inspection report § follow up on non-compliances after GMP inspection § Joint GMP inspections 11/23/2020 10

Achievements II Under the WHO PQ-EAC Inspection Collaboration Procedure with EAC NMRAs: q exchange of information including inspection schedules through a secure website q WHO support to training of GMP Inspectors through training courses q Inspectors from EAC Partner States NMRAs have participated in joint inspections as either observers or co-inspectors q Inspectors have used the prequalification program as a platform for capacity building at international level through participation in inspections as observers q. Inspectors from different NMRAs have participated in joint inspections within the region when Partner States NMRAs are inspecting facilities within their countries 11/23/2020 11

TFDA NDA ZFDA KPPB RWANDA 11/23/2020 12

Challenges ØLack of mutually recognized legal frame work encompassing all the Partner States reduces the speed at which the TWGs are moving. Each Partner State has its own laws and regulations that are independent ØAll the Partner States NMRAs are at a different levels of medicine regulation and as a result moving at the same speed is still a challenge 11/23/2020 13

Plan for the future ØPlan for July 2014 to June 2015: ØContinue with joint inspections among member states Ø twinning among the member states Ø develop formal training program for GMP ØParticipate in development of a legal framework for GMP ØContinuous, regular joint inspections with other international bodies, programs like the WHO prequalification program and other well resourced NMRAs ØCommon database and a platform for sharing information which will guide regulatory decisions 11/23/2020 14

11/23/2020 15

Thank you for listening kikulek@who. int katkikul@gmail. com 11/23/2020 16
 Self inspection gmp
Self inspection gmp Gmp types
Gmp types Global harmonization task force
Global harmonization task force Data harmonization best practices
Data harmonization best practices Grade harmonization
Grade harmonization Africa animism
Africa animism East african tea trade association
East african tea trade association Divergent boundaries
Divergent boundaries East african lion
East african lion Ministry of east african community affairs uganda
Ministry of east african community affairs uganda Whats a rift valley
Whats a rift valley What is the horizontal movement of air called
What is the horizontal movement of air called Laissez faire theory
Laissez faire theory East is east and west is west
East is east and west is west Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan






























