Applying Genomic Profiling to Precision Cancer Medicine in






- Slides: 14

Applying Genomic Profiling to Precision Cancer Medicine in Clinical Practice George D. Demetri MD Senior Vice-President for Experimental Therapeutics Dana-Farber Cancer Institute Professor of Medicine, Harvard Medical School Co-Director, Ludwig Center at Harvard Medical School Boston, Massachusetts USA george_demetri@dfci. harvard. edu



Challenges to Clinical Application of New Diagnostic Genomic Technologies in Cancer • Variations in testing • Lack of standards for conduct or interpretation of tumor testing • Lack of data (and overly enthusiastic hype) for value of genomic profiling of tumors • Lack of patient/payor/physician demand

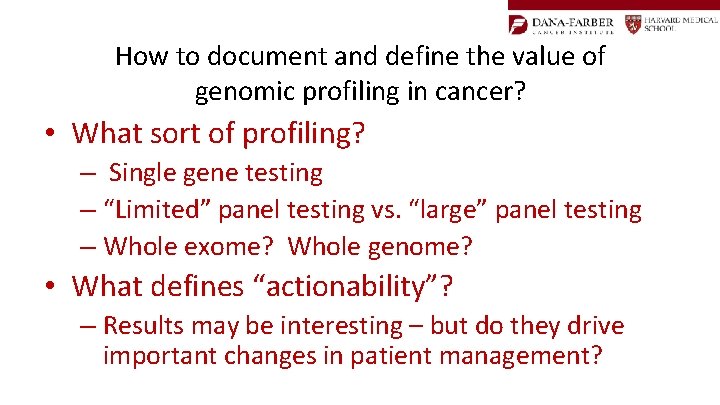
How to document and define the value of genomic profiling in cancer? • What sort of profiling? – Single gene testing – “Limited” panel testing vs. “large” panel testing – Whole exome? Whole genome? • What defines “actionability”? – Results may be interesting – but do they drive important changes in patient management?

Proving the value of genomic profiling in cancers • What drives demand for genomic profiling? • What determines the value of the most accurate diagnosis? – An available therapy? A change in prognosis? • How can we make accurate, large-scale profiling a reliable, inexpensive and necessary commodity?

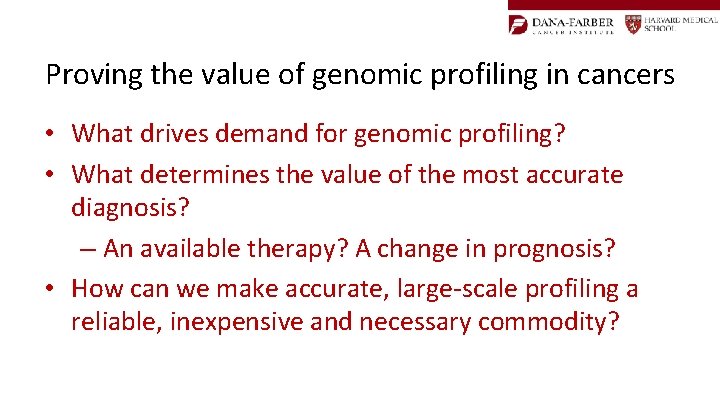
Complexity in Cancer: The GIST Example KIT 1 Dominant Mutation per patient – Site of mutation differs between patients PDGFRA Exon 9 (8%) - SENS Exon 11 (76%) - SENSITIVE Exon 13 (1%) - +/-SENS Exon 17 (1%) – RESISTANT TO TKI WILD TYPE in both KIT and PDGFRA (13%) – +/-RESISTANT TO TKIs Membrane Exon 12 (0. 3%) - SENSITIVE Cytoplasm Exon 18 (0. 6%) - RESISTANT

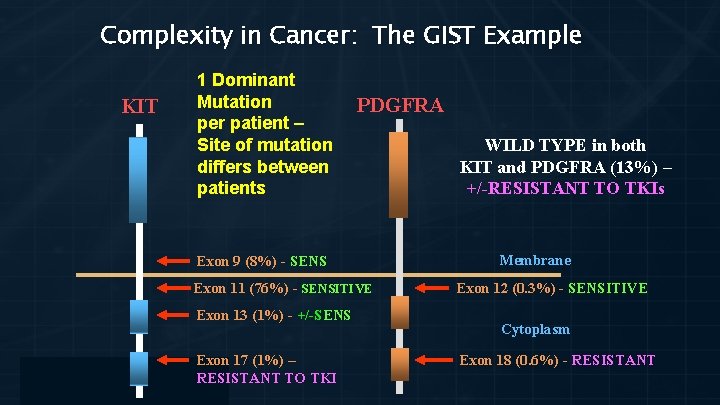
GIST with different mutations behave differently KIT mutant Exon 11 KIT mutant Exon 9 No KIT Mutation Different Genotypic and Structural Variants Fail Imatinib Therapy at Different Rates Heinrich, Corless, Fletcher, Demetri et al.

Patients Identify with Precision Medicine

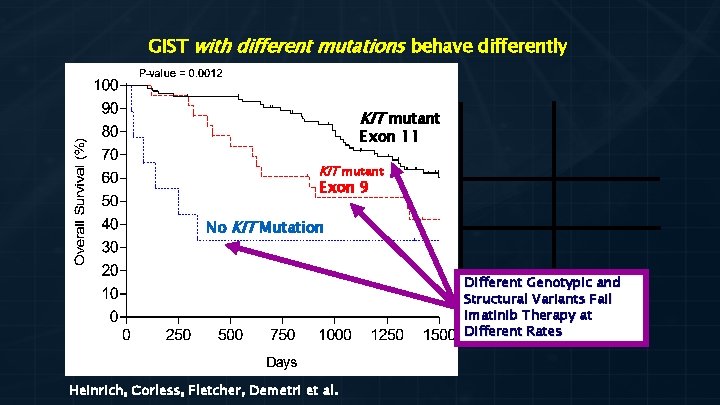
Clinically-Important Genomic Differences Between Individual Patients with GIST KIT mutation (75% to 80%) GIST PDGFRA mutation (approx 10%) SDH mutations or silencing (SDHA, SDHB, SDHC) (approx 10%) BRAF or NF 1 mutations (<2%) • SPECIFIC SUBTYPES of above mutations can also impact patient outcomes 1 – Point mutations in KIT exon 11 confer overall favorable prognosis – KIT mutations in exon 9 associated with poor prognosis – PDGFRA D 842 V mutation: good risk in primary GIST , worse outcomes in metastatic GIST 1. Corless CL, et al. Nat Rev Cancer. 2011; 11(12): 865 -878.

A Biblical Analogy • What is the minimum value for molecular profiling to become a necessary standard? • The story of Abraham negotiating with G_d about what level of ”righteousness” would save Sodom. – 50 ”righteous” people out of 100, 000? 25? 10? • 0. 0001% would be acceptable. • For 1. 7 M cancer diagnoses per year in US, need to change management in only 170 patient cases

Other Barriers to Optimal Use of Genomic Profiling • Education of physicians • Lack of (educated/rational) demand from patients • Indiscriminate interpretation of results – Not all BRAF mutations have the same therapeutic implications • Fear of legal action to obtain expensive drugs without adequate clinical justification

Summary on Status of Genomic Profiling to Enhance Meaningful Precision in Cancer Medicine • Feasibility of expansion for the best profiling technologies • Need large-scale access and rigorous analysis of impact • Fairness principles should apply (not just who is willing and able to pay for profiling) • Objective analytics on impact in clinical outcomes – Define important changes in patient care and outcomes

 Semi precision attachments
Semi precision attachments Bcd gösterimi
Bcd gösterimi Linear measuring instruments
Linear measuring instruments Precision medicine ecosystem
Precision medicine ecosystem Ethical issues in precision medicine
Ethical issues in precision medicine Martin tobin leicester
Martin tobin leicester Dr. martin tobin
Dr. martin tobin Genomic england
Genomic england Genomic
Genomic Genomic england
Genomic england Genomic imprinting definition
Genomic imprinting definition Anneke seller
Anneke seller Comparative genomic hybridization animation
Comparative genomic hybridization animation Genomic signal processing
Genomic signal processing Genomic equivalence definition
Genomic equivalence definition