Apple Experiment Come down and get an apple










- Slides: 31

Apple Experiment Come down and get an apple and a slice of lemon. When you get back to your seat: 1. Take a big bite of your delicious apple. 2. Immediately squeeze lemon juice over the apple flesh that is now exposed from the bite. 3. IMPORTANT! Don’t get lemon juice all over the apple. Make sure that it is ONLY on the area that you just bit! 4. Set the lemon aside and wipe any lemon juice off of your hands with a napkin. 5. Take another bite from the opposite side of your apple. 6. Set your apple aside.

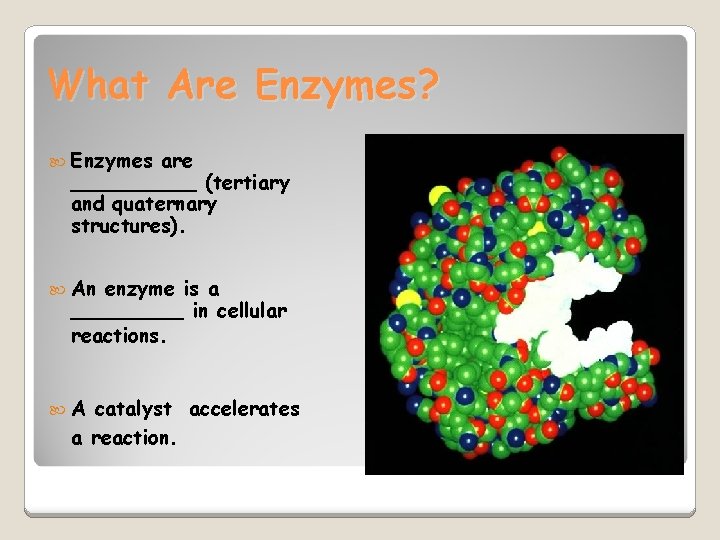
What Are Enzymes? Enzymes are _____ (tertiary and quaternary structures). An enzyme is a _____ in cellular reactions. A catalyst accelerates a reaction.

Enzymes Are ____ for what they will catalyze Are _____ Usually end in -_____ -Sucrase -Lactase -Maltase

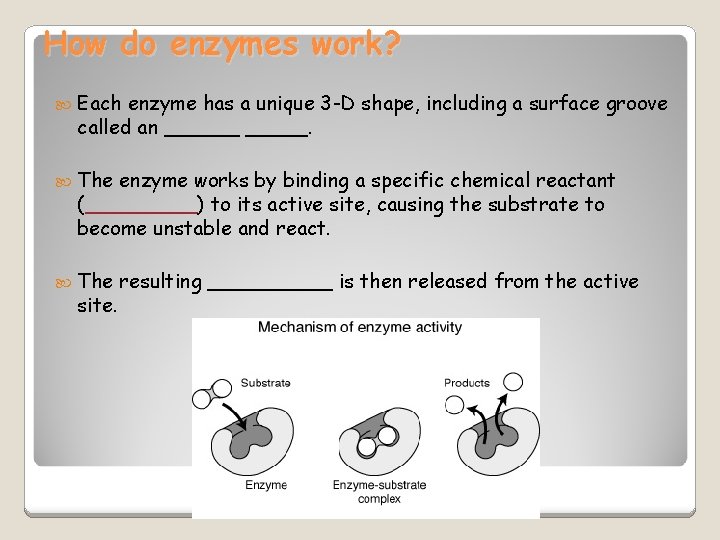
How do enzymes work? Each enzyme has a unique 3 -D shape, including a surface groove called an ______. The enzyme works by binding a specific chemical reactant (_____) to its active site, causing the substrate to become unstable and react. The site. resulting _____ is then released from the active

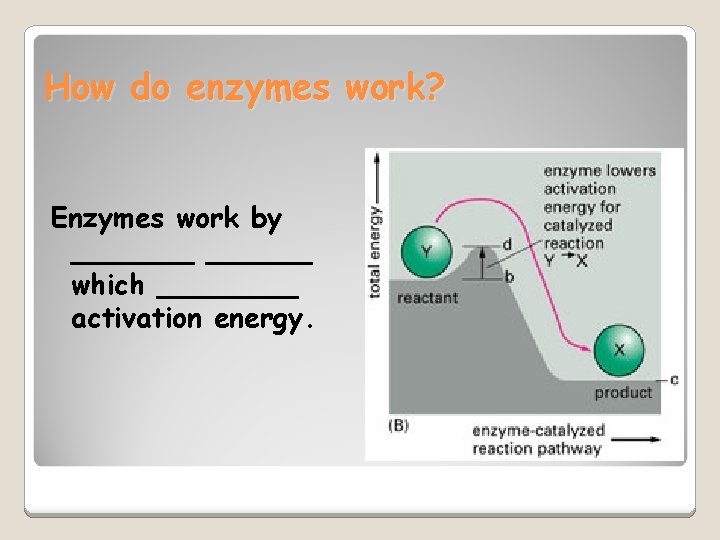
How do enzymes work? Enzymes work by _______ which ____ activation energy.

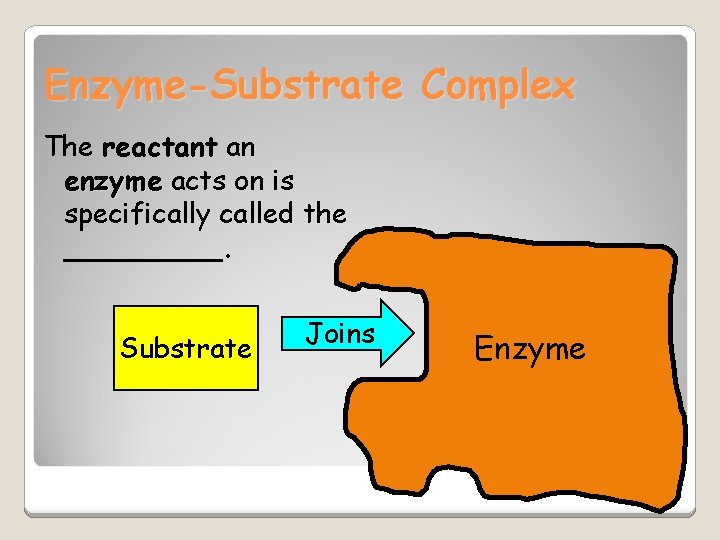
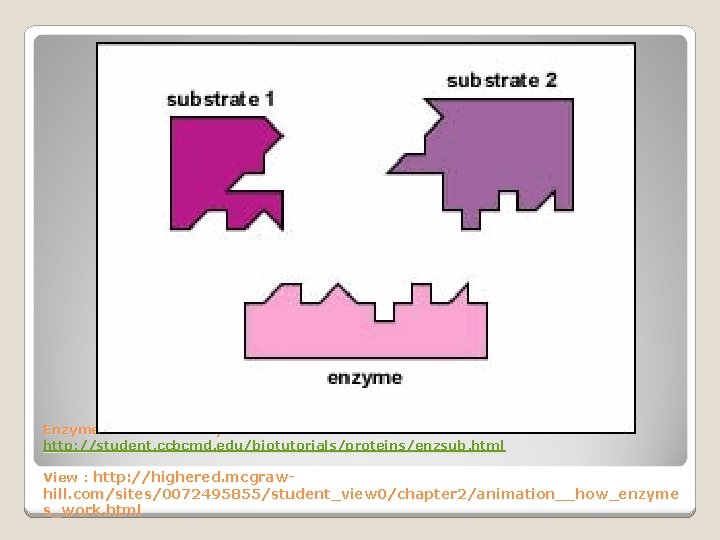
Enzyme-Substrate Complex The reactant an enzyme acts on is specifically called the _____. Substrate Joins Enzyme

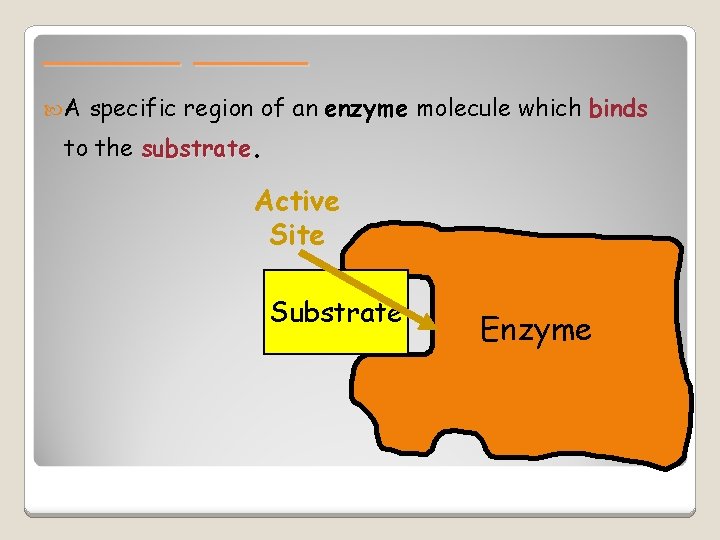
______ A specific region of an enzyme molecule which binds to the substrate. Active Site Substrate Enzyme

Shape of a Protein An enzyme fits with its substrate like a ____ and ____.

Enzyme. Animation : Gary E. Kaiser http: //student. ccbcmd. edu/biotutorials/proteins/enzsub. html View : http: //highered. mcgraw- hill. com/sites/0072495855/student_view 0/chapter 2/animation__how_enzyme s_work. html

Enzymes are ________ in the reactions they catalyze. Think of them as tiny machines in manufacturing. The more machines, the faster the accumulation of _____. Image: Wine Bottling : www. morrison-chs. com/timingscrews/index. html Wine Vats: www. lymebaywinery. co. uk/pages/about_us. htm


4 levels of Protein Structure

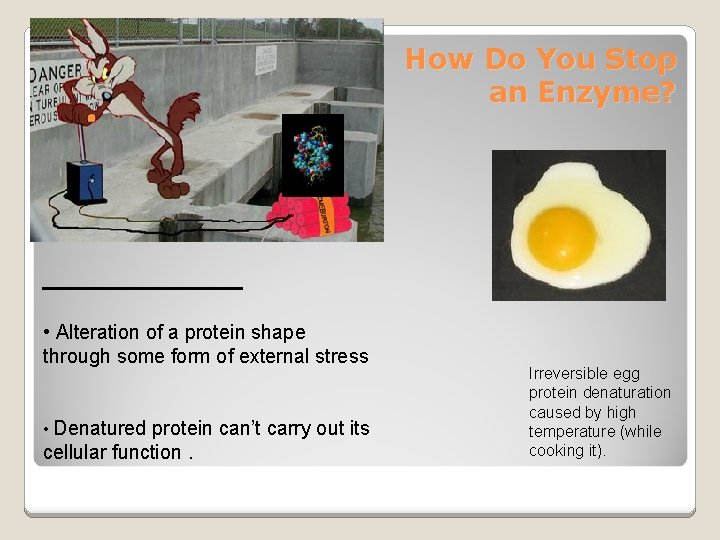
How Do You Stop an Enzyme? ________ • Alteration of a protein shape through some form of external stress • Denatured protein can’t carry out its cellular function. Irreversible egg protein denaturation caused by high temperature (while cooking it).

Factors Affecting Enzyme Activity Temperature p. H Cofactors Inhibitors & Coenzymes

Temperature & p. H Think about what kind of cell or organism an enzyme may work in… Temperatures far above the normal range _____ enzymes (This is why very high fevers are so dangerous. They can cook the body’s proteins) Most enzymes work best near _____ p. H (6 to 8).

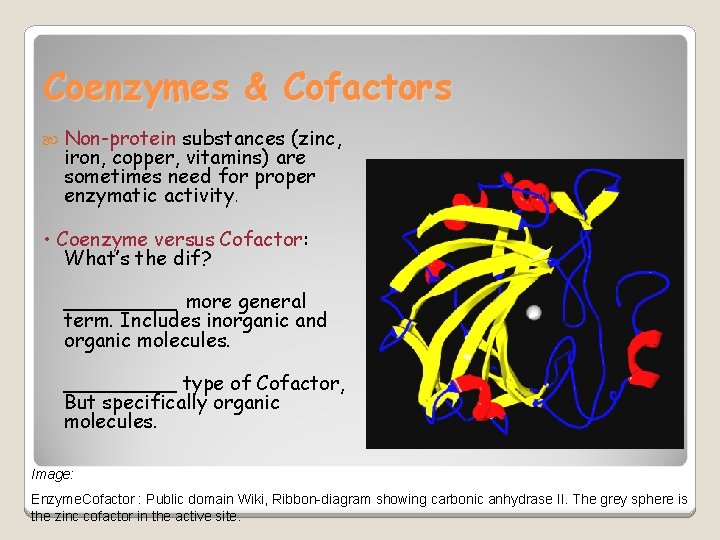
Coenzymes & Cofactors Non-protein substances (zinc, iron, copper, vitamins) are sometimes need for proper enzymatic activity. • Coenzyme versus Cofactor: What’s the dif? _____ more general term. Includes inorganic and organic molecules. _____ type of Cofactor, But specifically organic molecules. Image: Enzyme. Cofactor : Public domain Wiki, Ribbon-diagram showing carbonic anhydrase II. The grey sphere is the zinc cofactor in the active site.

Coenzyme : Vitamin B 12 Example: Most _____ are coenzymes essential in helping move atoms between molecules in the formation of carbohydrates, fats, and proteins. • Exclusively synthesized by ______ (found primarily in meat, eggs and dairy products). Image: Vitamin. B 12 : NIH, Public Domain www. nlm. nih. gov/. . . /ency/imagepages/19516. htm

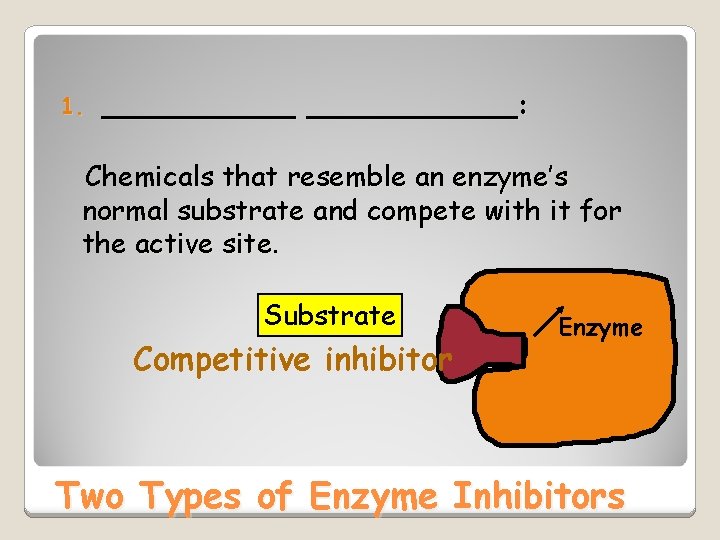
1. ____________: Chemicals that resemble an enzyme’s normal substrate and compete with it for the active site Substrate Competitive inhibitor Enzyme Two Types of Enzyme Inhibitors

1. Competitive inhibitors: Resemble an enzyme’s normal substrate and compete with it for the active site Image: Competitive Inhibition : www-biol. paisley. ac. uk/. . . /chapter 3_2. html

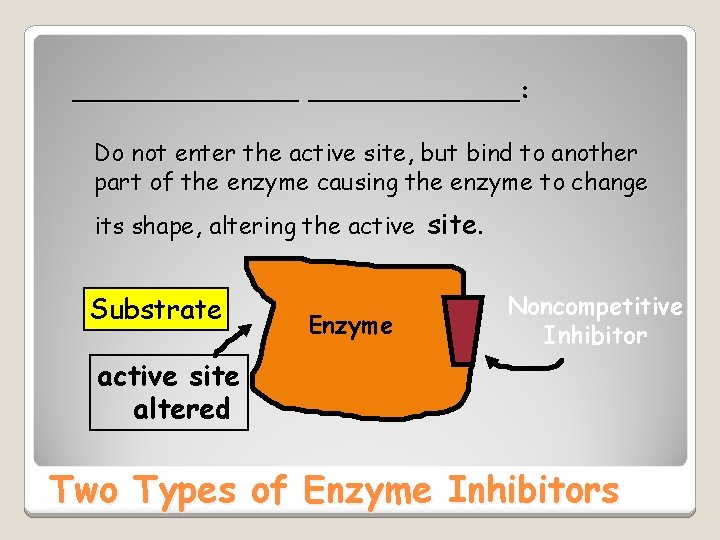
________: Do not enter the active site, site but bind to another part of the enzyme causing the enzyme to change its shape, shape altering the active site Substrate Enzyme Noncompetitive Inhibitor active site altered Two Types of Enzyme Inhibitors

Enzyme Inhibitors Blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance. Many _____ are enzyme inhibitors. Enzyme inhibitors are also used as _____ and _____. Images Dead Bug : www. kansas. gov/help_center/user_testing. html Prescription Drugs : www. patentdocs. us/. . . /08/by-kevin-e-noon. html

Regulation of Enzyme Activity

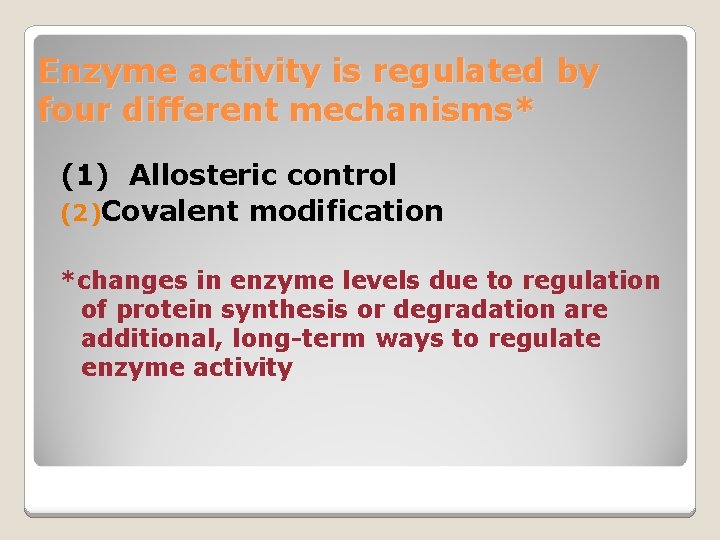
Enzyme activity is regulated by four different mechanisms* (1) Allosteric control (2)Covalent modification *changes in enzyme levels due to regulation of protein synthesis or degradation are additional, long-term ways to regulate enzyme activity

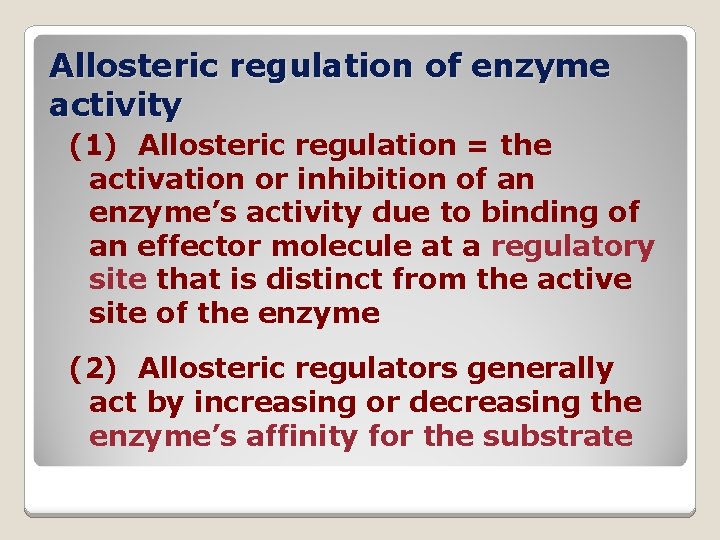
Allosteric regulation of enzyme activity (1) Allosteric regulation = the activation or inhibition of an enzyme’s activity due to binding of an effector molecule at a regulatory site that is distinct from the active site of the enzyme (2) Allosteric regulators generally act by increasing or decreasing the enzyme’s affinity for the substrate

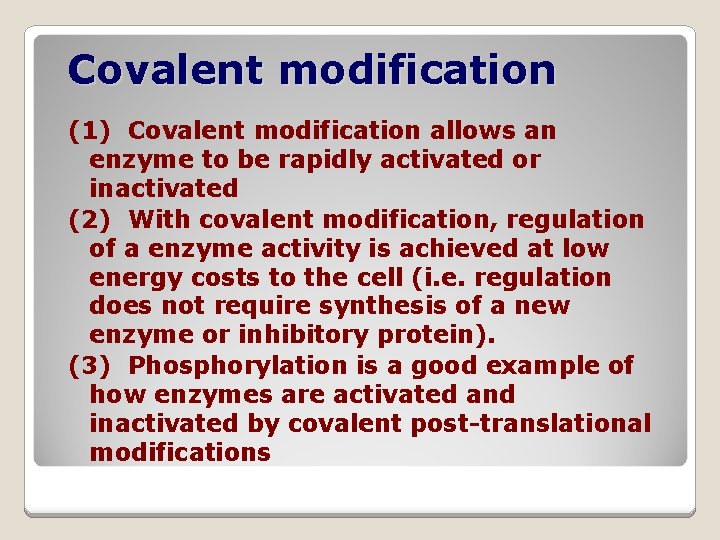
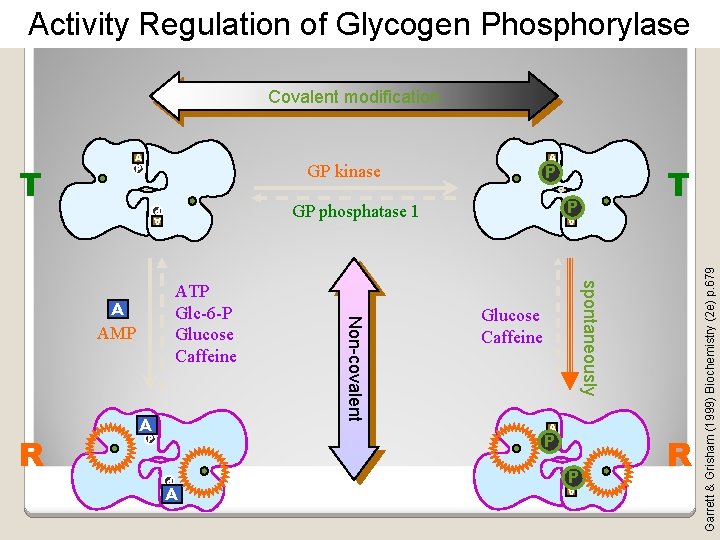
Covalent modification (1) Covalent modification allows an enzyme to be rapidly activated or inactivated (2) With covalent modification, regulation of a enzyme activity is achieved at low energy costs to the cell (i. e. regulation does not require synthesis of a new enzyme or inhibitory protein). (3) Phosphorylation is a good example of how enzymes are activated and inactivated by covalent post-translational modifications

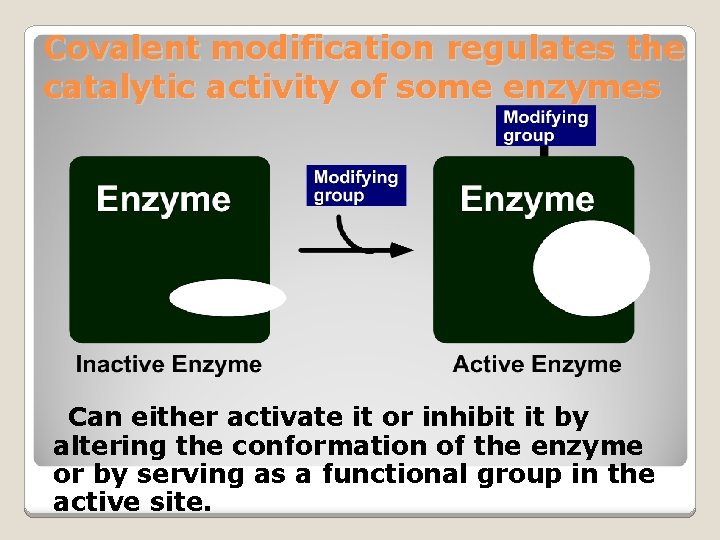
Covalent modification regulates the catalytic activity of some enzymes Can either activate it or inhibit it by altering the conformation of the enzyme or by serving as a functional group in the active site.

Summary of regulatory mechanisms (1) Allosteric regulation ATP activation/CTP inhibition of ATCase sigmoidal kinetics c. AMP activation of c. AMP-dependent protein kinase (2) Reversible covalent modification Phosphorylation Ser/Thr protein kinases, Tyr kinases, kinase cascades

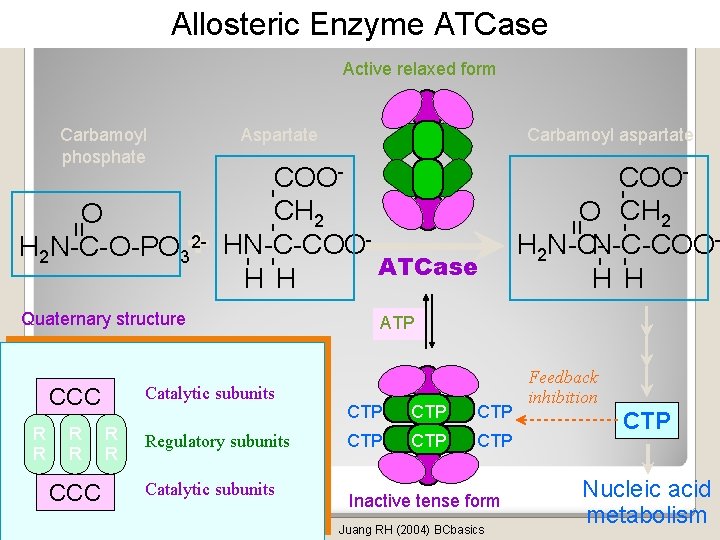
Allosteric Enzyme ATCase Active relaxed form Catalytic subunits R R CCC R R Regulatory subunits Catalytic subunits COOO CH 2 N-C-COOH 2 N-CH H - - - COOCH 2 HN-C-COOATCase H H Quaternary structure CCC Carbamoyl aspartate = = O 2 H 2 N-C-O-PO 3+ Aspartate - - - Carbamoyl phosphate ATP CTP CTP CTP Inactive tense form Juang RH (2004) BCbasics Feedback inhibition CTP Nucleic acid metabolism

Sigmoidal vo Curve Effect Sigmoidal curve Noncooperative (Hyperbolic) ATP Cooperative (Sigmoidal) Positive effector (ATP) brings sigmoidal curve back to hyperbolic Negative effector (CTP) keeps vo Exaggeration of sigmoidal curve yields a drastic zigzag line that shows the On/Off point clearly Consequently, Allosteric enzyme can sense the concentration of the environment and adjust its activity Off On [Substrate] Juang RH (2004) BCbasics

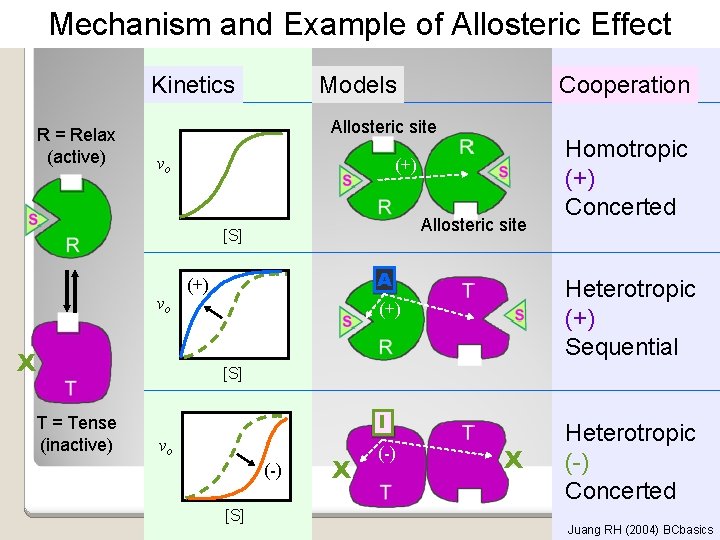
Mechanism and Example of Allosteric Effect Kinetics R = Relax (active) Models Allosteric site vo (+) Allosteric site [S] vo X T = Tense (inactive) Cooperation A (+) Homotropic (+) Concerted Heterotropic (+) Sequential (+) [S] I vo (-) [S] X (-) X Heterotropic (-) Concerted Juang RH (2004) BCbasics

Activity Regulation of Glycogen Phosphorylase Covalent modification A P AA spontaneously AMP Non-covalent A Glucose Caffeine A P P P A P A P R P GP phosphatase 1 ATP Glc-6 -P Glucose Caffeine T P GP kinase A P T A P R Garrett & Grisham (1999) Biochemistry (2 e) p. 679 A P