Analytical development challenges in homeopathy detect and quantify












- Slides: 34

Analytical development challenges in homeopathy: detect and quantify quality and toxicological markers AAHP - Baltimore - 27 -28 June 2019 Stéphanie Chanut, Pharmaceutical Development Laboratory manager, Boiron 1

Purpose of the presentation § Identification and quantification of relevant compounds using appropriate techniques (TLC, GC, UV spectrophometry, titrations) § Toxic constituents § Characteristics constituents/major constituents § At which step ? Challenges § Contents in the tincture, in the drug product § Variability of herbal substance: homogenous/heterogenous contents § Stability of the constituents § Availability of reference substances § Method appropriate for routine applications Approach to guarantee the quality and the safety of homeopathic drug products 2

Agenda Regulatory backgrounds of homeopathic drug products Manufacture of homeopathic finished products and purpose of the analytical development Markers Detection, identification and quantification of markers in the tincture Analytical techniques from tincture to dilutions and finished products Example of the detection of alkaloids in tincture, dilutions and finished product Example of the detection of other characteristic consituents in tinctures and finished product Conclusion 3

Regulatory background of homeopathic drug products FDA framework – GMP – Same requirements concerning approval, adulteration and misbranding as any other products – No specific premarket approval if products compliant with CPG 400 – Major concern: patient safety – Draft guideline with a risk-based enforcement approach • Example of products for vulnerable populations (infants and children) containing ingredients with high safety concerns (Belladonna, Nux vomica) • Example of products intended for prevention or treatment of serious diseases HPUS – Monographs with requirements for starting materials and tinctures – Monograph with general requirements for manufacture of tinctures, dilutions, pharmaceutical forms USP – General requirements for pharmaceutical forms 4

Regulatory background of homeopathic drug products WHO framework – Guarantee the quality and safety of homeopathic drug products – Compliance with GMP – Identification and quantification of active and toxic substances in the final product : not always possible given the level of dilutions – Determination of toxic constituent contents/markers content in • Herbal, animal, chemical and mineral raw materials when applicable • Tincture – Raw materials, tinctures, diluents and excipients comply with pharmacopoeia 5

Regulatory background of homeopathic drug products European framework – Directive 2003/63/EC • If possible and if cannot be on the final drug product given dilution levels, identification / assay of toxic constituents required on starting material – HMPWG[1] guidelines • Compliant with CHMP [2] /HMPC [3] quality guidelines • Analytical technique for raw materials, homeopathic stocks and dilutions including – Chromatographic techniques suited to the composition – Assay of main ingredients if applicable – CHMP/HMPC quality guidelines for products manufactured from herbal substance • Introduce the notion of “analytical markers” where compounds with therapeutic activity/active markers are unknown [1] HMPWG: Homeopathic Medicinal Product Working Group (European Working Group oh Health Authorities ) [2] [3] CHMP: Committee for Medicinal Products for Human Use HMPC: Committee on Herbal Medicinal Products 6

Regulatory background of homeopathic drug products European framework – Pharmacopoeia monographs describing • General European Pharmacopoeia monographs – Homeopathic preparations (1038) – Methods of preparation of homoeopathic stocks and potentisation (2371) – Mother tinctures for homoeopathic preparations (2029) – Herbal drugs for homoeopathic preparations (2045) – Monographs for dosage forms specific to homeopathic drug products (pilules) • Specific monographs in Ph. Eur. , Fr. Ph. , HAB, defining the requirements to control raw materials and tincture and specifying different parameters to check among which : – TLC profile – Assay of toxic/characteristic constituents 7

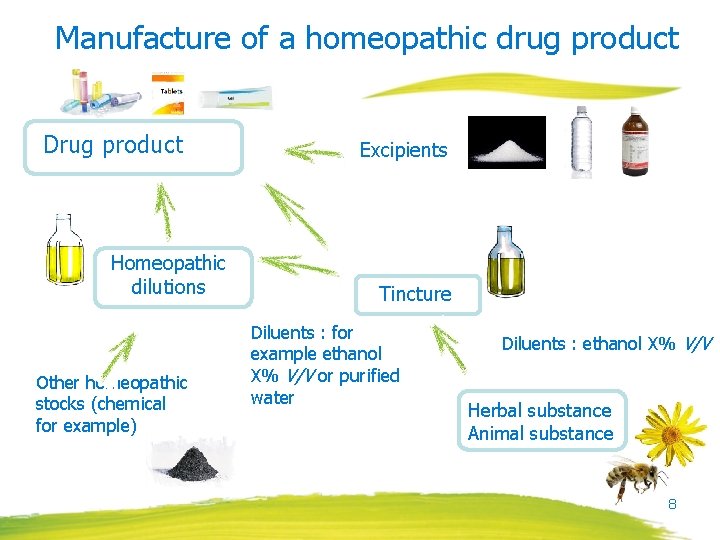
Manufacture of a homeopathic drug product Drug product Homeopathic dilutions Other homeopathic stocks (chemical for example) Excipients Tincture Diluents : for example ethanol X% V/V or purified water Diluents : ethanol X% V/V Herbal substance Animal substance 8

Markers: phytochemical constituents present in herbal substance and in tincture in concentrations enabling their quantification – Toxicological markers • Phytochemical constituents known for their toxicity (example of alkaloids). • No link with a therapeutic activity • Quantified in tincture for a safety evaluation purpose with a maximum limit – Quality markers (= analytical markers) • Phytochemical constituents present in the plant according to bibliographic data for which no toxicity is known • Contents in herbal substance and tincture enabling identification and sometimes quantification • No link with a therapeutic activity • No link with any toxicity • Analytical markers quantified in tincture for quality evaluation purpose 9

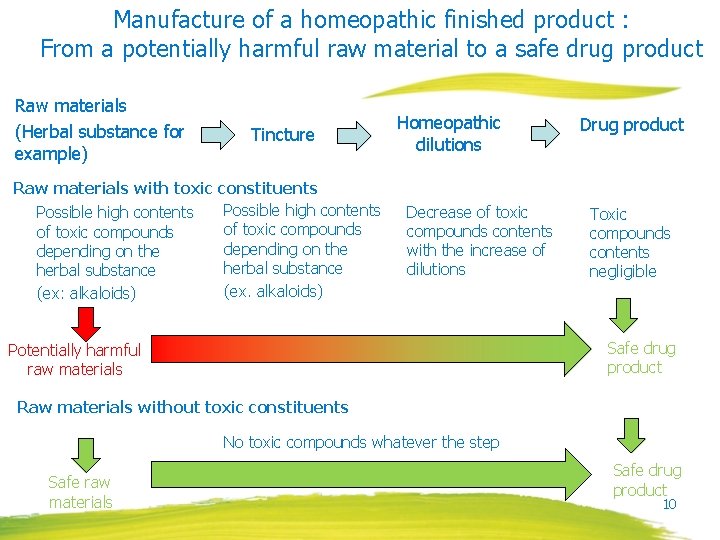
Manufacture of a homeopathic finished product : From a potentially harmful raw material to a safe drug product Raw materials (Herbal substance for example) Tincture Raw materials with toxic constituents Possible high contents of toxic compounds depending on the herbal substance (ex. alkaloids) (ex: alkaloids) Homeopathic dilutions Decrease of toxic compounds contents with the increase of dilutions Drug product Toxic compounds contents negligible Safe drug product Potentially harmful raw materials Raw materials without toxic constituents No toxic compounds whatever the step Safe raw materials Safe drug product 10

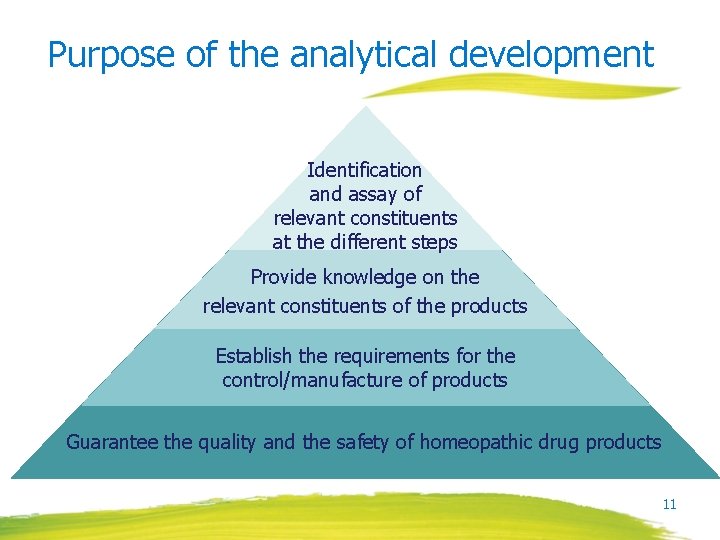
Purpose of the analytical development Identification and assay of relevant constituents at the different steps Provide knowledge on the relevant constituents of the products Establish the requirements for the control/manufacture of products Guarantee the quality and the safety of homeopathic drug products 11

Constituents of the raw materials Bibliographical research on the herbal substance Phytochemical composition of the herbal substance with focus on – Toxic constituents: alkaloids in Belladonna, Nux vomica – Characteristics constituents: flavonoids in Allium cepa • Preferentially secondary botanical metabolites • Sometimes primary botanical metabolites may be used if characteristics of the plant part (exemple of fatty acids in seeds) 12

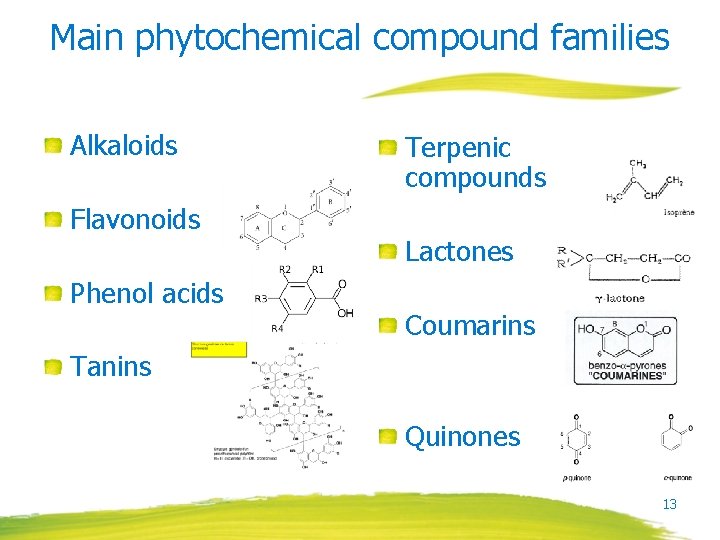
Main phytochemical compound families Alkaloids Flavonoids Phenol acids Terpenic compounds Lactones Coumarins Tanins Quinones 13

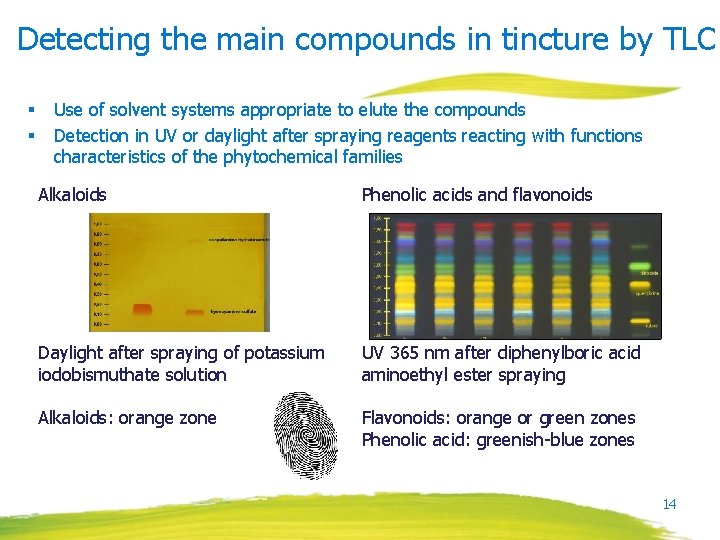
Detecting the main compounds in tincture by TLC § § Use of solvent systems appropriate to elute the compounds Detection in UV or daylight after spraying reagents reacting with functions characteristics of the phytochemical families Alkaloids Phenolic acids and flavonoids Daylight after spraying of potassium iodobismuthate solution UV 365 nm after diphenylboric acid aminoethyl ester spraying Alkaloids: orange zone Flavonoids: orange or green zones Phenolic acid: greenish-blue zones 14

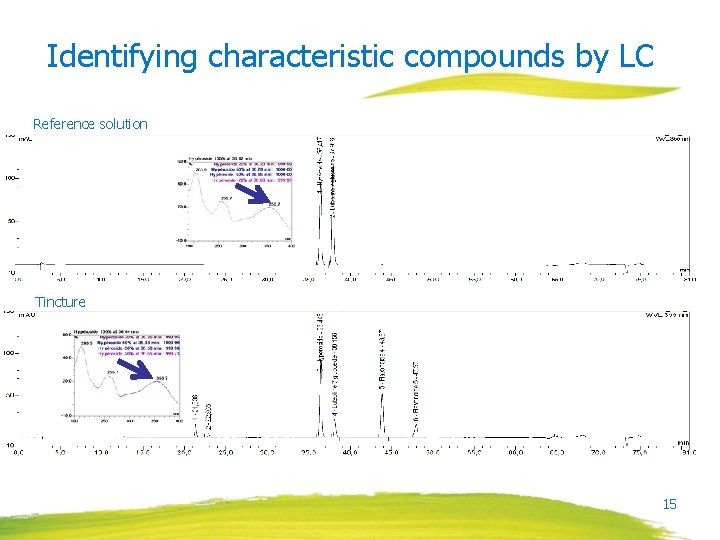
Identifying characteristic compounds by LC Reference solution Tincture 15

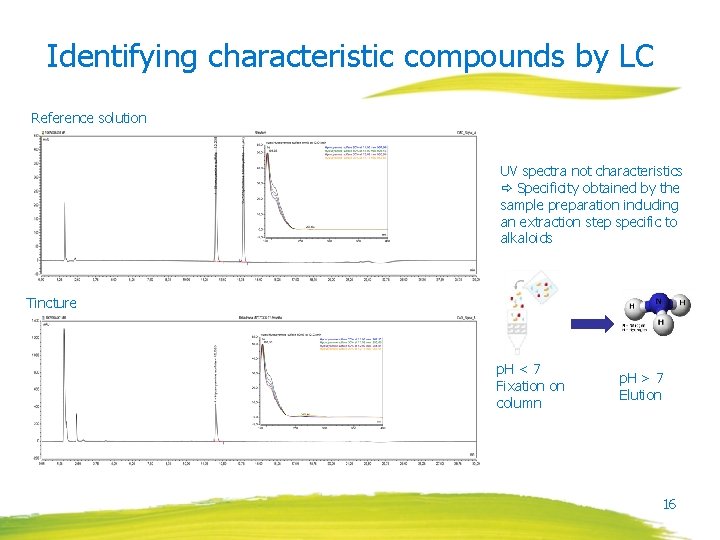
Identifying characteristic compounds by LC Reference solution UV spectra not characteristics Specificity obtained by the sample preparation including an extraction step specific to alkaloids Tincture p. H < 7 Fixation on column p. H > 7 Elution 16

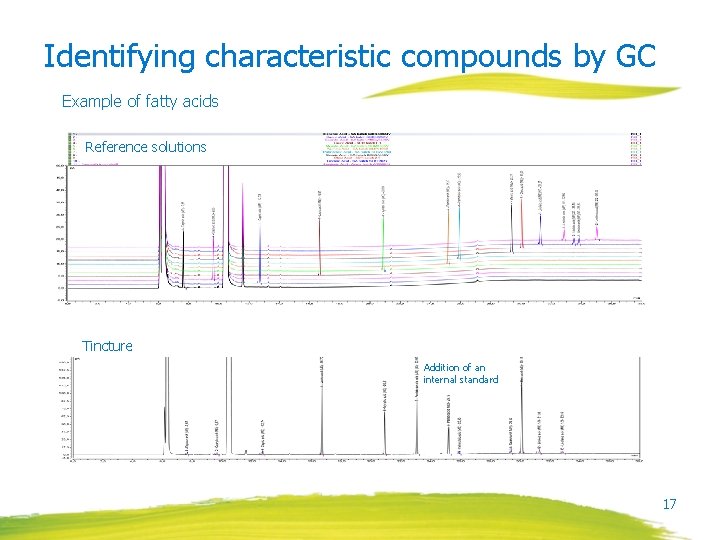
Identifying characteristic compounds by GC Example of fatty acids Reference solutions Tincture Addition of an internal standard 17

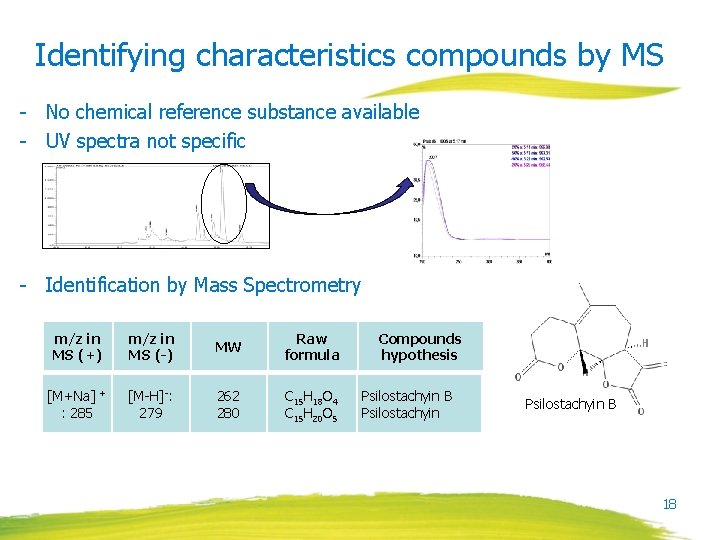
Identifying characteristics compounds by MS - No chemical reference substance available - UV spectra not specific - Identification by Mass Spectrometry m/z in MS (+) m/z in MS (-) MW Raw formula [M+Na] + : 285 [M-H]-: 279 262 280 C 15 H 18 O 4 C 15 H 20 O 5 Compounds hypothesis Psilostachyin B 18

Establishing requirements for tinctures § Defining TLC profile characteristic of the tincture enabling identification and quality evaluation (routine control, process validation and stability) § Choice of assay markers : § Toxicological and/or quality markers § Setting analytical methods § Validation of the analytical methods according to ICH Q 2(R 1) recommendations § Results on several batches (3 – 6 batches generally) to assess variability and define appropriate acceptance criteria 19

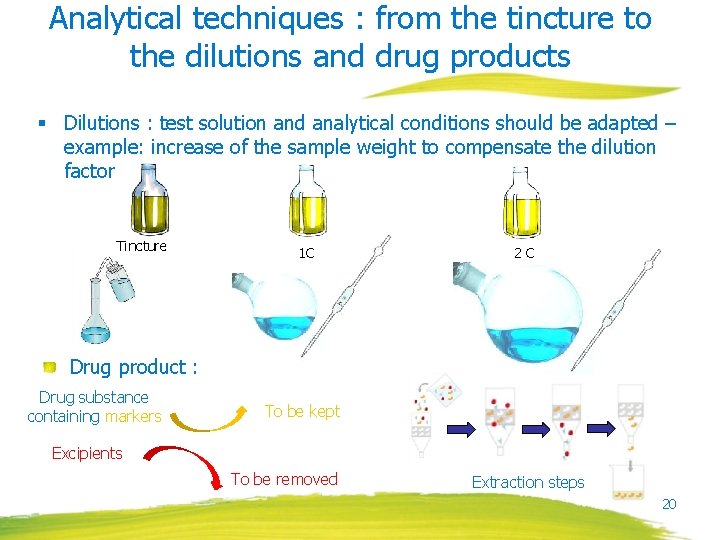
Analytical techniques : from the tincture to the dilutions and drug products § Dilutions : test solution and analytical conditions should be adapted – example: increase of the sample weight to compensate the dilution factor Tincture 1 C 2 C Drug product : Drug substance containing markers To be kept Excipients To be removed Extraction steps 20

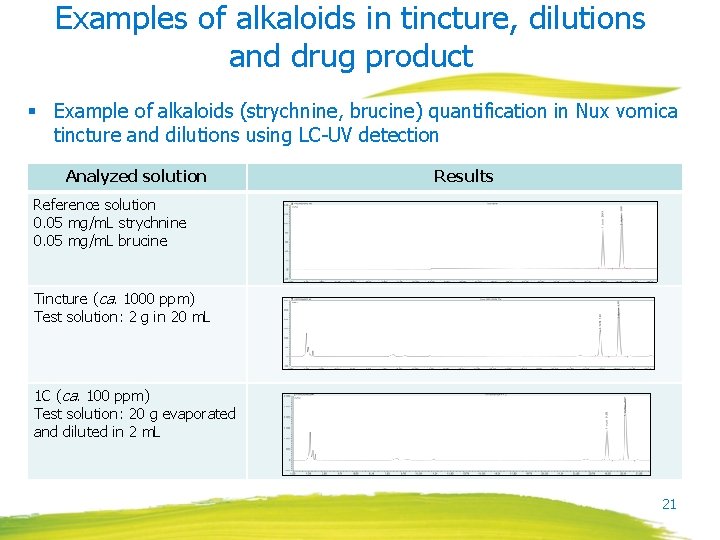
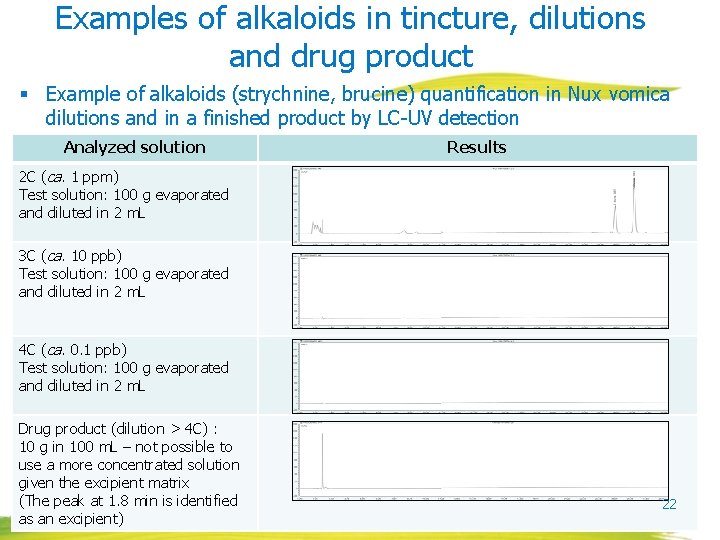
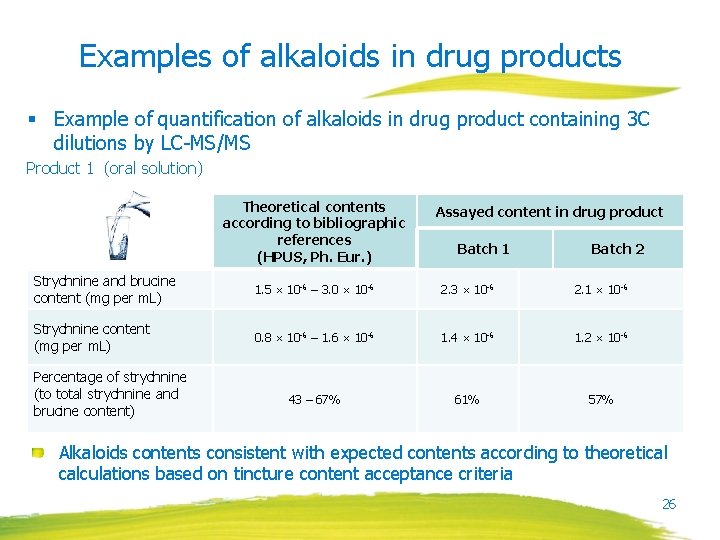
Examples of alkaloids in tincture, dilutions and drug product § Example of alkaloids (strychnine, brucine) quantification in Nux vomica tincture and dilutions using LC-UV detection Analyzed solution Results Reference solution 0. 05 mg/m. L strychnine 0. 05 mg/m. L brucine Tincture (ca. 1000 ppm) Test solution: 2 g in 20 m. L 1 C (ca. 100 ppm) Test solution: 20 g evaporated and diluted in 2 m. L 21

Examples of alkaloids in tincture, dilutions and drug product § Example of alkaloids (strychnine, brucine) quantification in Nux vomica dilutions and in a finished product by LC-UV detection Analyzed solution Results 2 C (ca. 1 ppm) Test solution: 100 g evaporated and diluted in 2 m. L 3 C (ca. 10 ppb) Test solution: 100 g evaporated and diluted in 2 m. L 4 C (ca. 0. 1 ppb) Test solution: 100 g evaporated and diluted in 2 m. L Drug product (dilution > 4 C) : 10 g in 100 m. L – not possible to use a more concentrated solution given the excipient matrix (The peak at 1. 8 min is identified as an excipient) 22

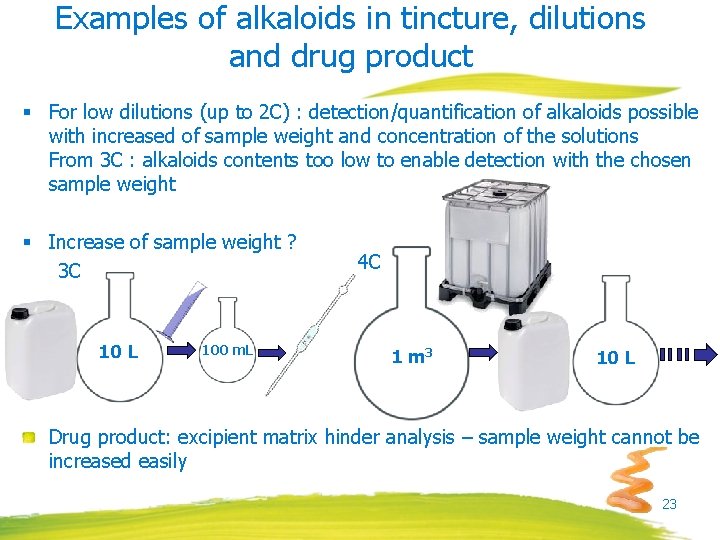
Examples of alkaloids in tincture, dilutions and drug product § For low dilutions (up to 2 C) : detection/quantification of alkaloids possible with increased of sample weight and concentration of the solutions From 3 C : alkaloids contents too low to enable detection with the chosen sample weight § Increase of sample weight ? 3 C 10 L 100 m. L 4 C 1 m 3 10 L Drug product: excipient matrix hinder analysis – sample weight cannot be increased easily 23

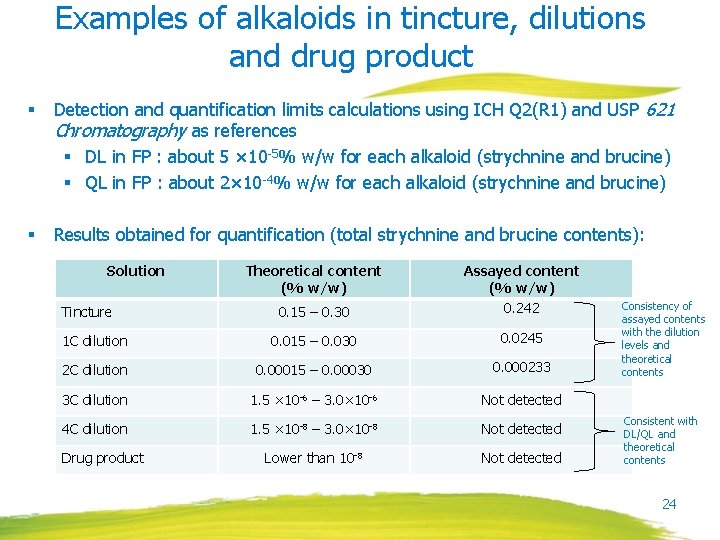
Examples of alkaloids in tincture, dilutions and drug product § Detection and quantification limits calculations using ICH Q 2(R 1) and USP 621 Chromatography as references § DL in FP : about 5 × 10 -5% w/w for each alkaloid (strychnine and brucine) § QL in FP : about 2× 10 -4% w/w for each alkaloid (strychnine and brucine) § Results obtained for quantification (total strychnine and brucine contents): Solution 0. 15 – 0. 30 Assayed content (% w/w) 0. 242 1 C dilution 0. 015 – 0. 030 0. 0245 2 C dilution 0. 00015 – 0. 00030 0. 000233 3 C dilution 1. 5 × 10 -6 – 3. 0× 10 -6 Not detected 4 C dilution 1. 5 × 10 -8 – 3. 0× 10 -8 Not detected Lower than 10 -8 Not detected Tincture Drug product Theoretical content (% w/w) Consistency of assayed contents with the dilution levels and theoretical contents Consistent with DL/QL and theoretical contents 24

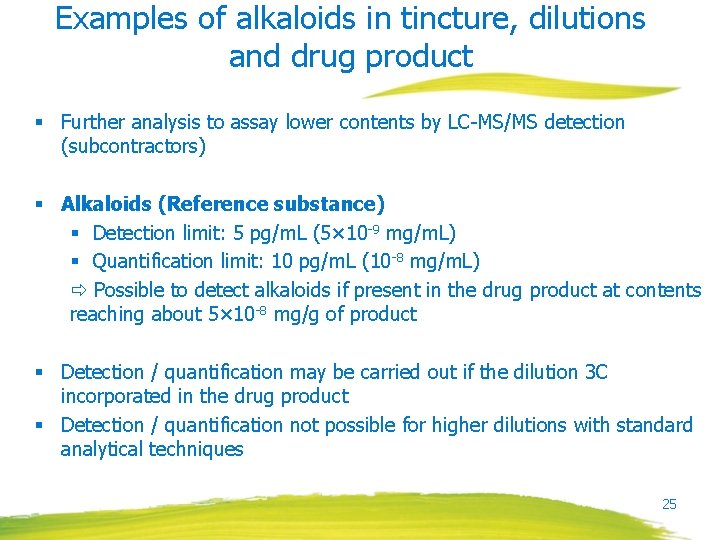
Examples of alkaloids in tincture, dilutions and drug product § Further analysis to assay lower contents by LC-MS/MS detection (subcontractors) § Alkaloids (Reference substance) § Detection limit: 5 pg/m. L (5× 10 -9 mg/m. L) § Quantification limit: 10 pg/m. L (10 -8 mg/m. L) Possible to detect alkaloids if present in the drug product at contents reaching about 5× 10 -8 mg/g of product § Detection / quantification may be carried out if the dilution 3 C incorporated in the drug product § Detection / quantification not possible for higher dilutions with standard analytical techniques 25

Examples of alkaloids in drug products § Example of quantification of alkaloids in drug product containing 3 C dilutions by LC-MS/MS Product 1 (oral solution) Theoretical contents according to bibliographic references (HPUS, Ph. Eur. ) Assayed content in drug product Batch 1 Batch 2 Strychnine and brucine content (mg per m. L) 1. 5 × 10 -6 – 3. 0 × 10 -6 2. 3 × 10 -6 2. 1 × 10 -6 Strychnine content (mg per m. L) 0. 8 × 10 -6 – 1. 6 × 10 -6 1. 4 × 10 -6 1. 2 × 10 -6 43 – 67% 61% 57% Percentage of strychnine (to total strychnine and brucine content) Alkaloids contents consistent with expected contents according to theoretical calculations based on tincture content acceptance criteria 26

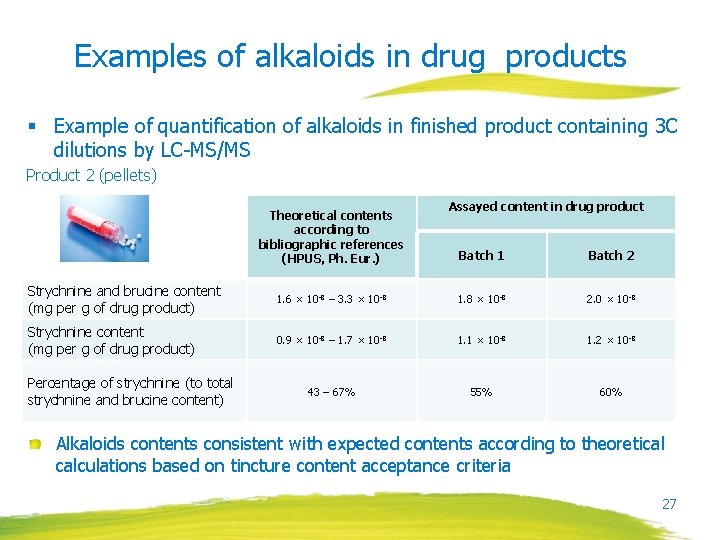
Examples of alkaloids in drug products § Example of quantification of alkaloids in finished product containing 3 C dilutions by LC-MS/MS Product 2 (pellets) Theoretical contents according to bibliographic references (HPUS, Ph. Eur. ) Assayed content in drug product Batch 1 Batch 2 Strychnine and brucine content (mg per g of drug product) 1. 6 × 10 -8 – 3. 3 × 10 -8 1. 8 × 10 -8 2. 0 × 10 -8 Strychnine content (mg per g of drug product) 0. 9 × 10 -8 – 1. 7 × 10 -8 1. 1 × 10 -8 1. 2 × 10 -8 43 – 67% 55% 60% Percentage of strychnine (to total strychnine and brucine content) Alkaloids contents consistent with expected contents according to theoretical calculations based on tincture content acceptance criteria 27

Examples of alkaloids in drug products § Example of quantification of alkaloids in finished product containing 3 C dilutions by LC-MS/MS Product 3 (syrup) - Expected content: 10 -7 mg/g of finished product - Unspecificity of the method : interference of matrix with the internal standard reaching more than 20% of the expected content - Linearity not compliant - Relative standard deviations (repeatability and intermediate precision) higher than 20% (for both reference solutions and finished product) No content can be stated based on finished product testing Results obtained for the tincture are thus the most appropriate to define the alkaloids contents in the finished product 28

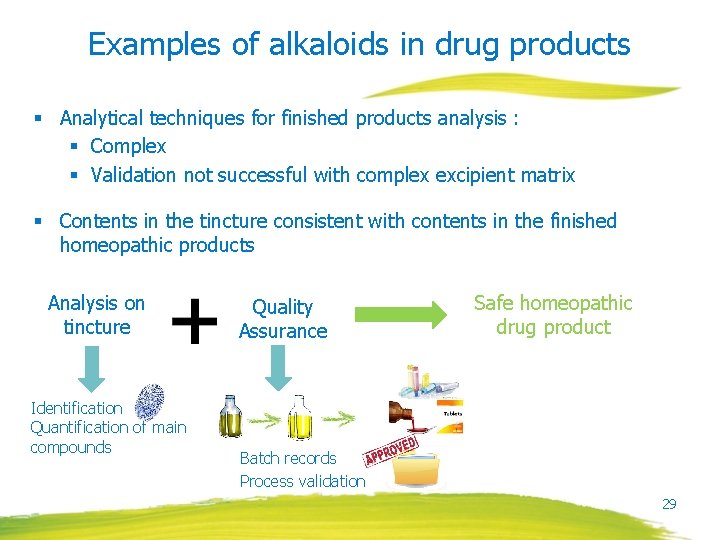
Examples of alkaloids in drug products § Analytical techniques for finished products analysis : § Complex § Validation not successful with complex excipient matrix § Contents in the tincture consistent with contents in the finished homeopathic products Analysis on tincture Identification Quantification of main compounds Quality Assurance Safe homeopathic drug product Batch records Process validation 29

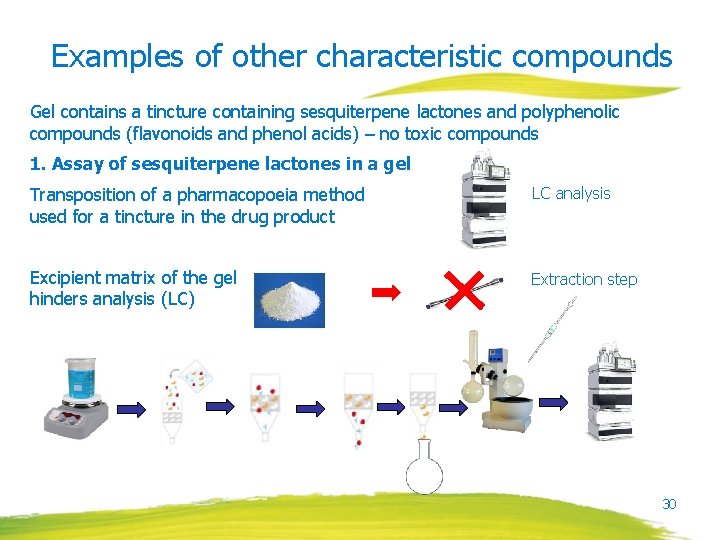
Examples of other characteristic compounds Gel contains a tincture containing sesquiterpene lactones and polyphenolic compounds (flavonoids and phenol acids) – no toxic compounds 1. Assay of sesquiterpene lactones in a gel Transposition of a pharmacopoeia method used for a tincture in the drug product LC analysis Excipient matrix of the gel hinders analysis (LC) Extraction step 30

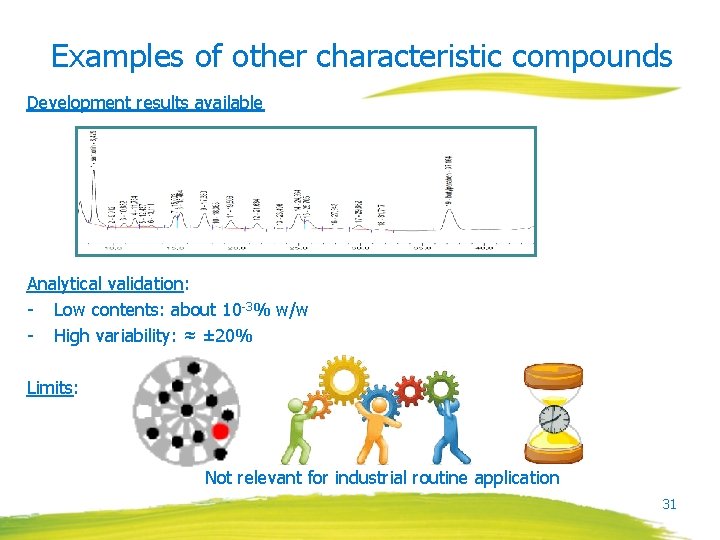
Examples of other characteristic compounds Development results available Analytical validation: - Low contents: about 10 -3% w/w - High variability: ≈ ± 20% Limits: Not relevant for industrial routine application 31

Examples of other characteristic compounds 2. Assay of total polyphenolic compounds in a gel § Global assay by UV-visible spectrophotometry § Dilution of the gel and hydrolysis in acid medium Phenols Phosphomolybdotungstic reagent Blue complex • Less impact of the excipient matrix if no absorption at the chosen wavelength • Higher content: about 10 -2% w/w • Lower variability: ≤ 5% • Assay helpful for process validation and stability evaluation 32

Conclusion § Importance to guarantee the quality and the safety of the homeopathic drug product throughout the manufacturing process from the starting material to the finished product § According to development data, when the manufacturing process is mastered (quality assurance) : contents assayed on dilutions and drug products are consistent with those calculated from data obtained on the tincture § Tincture : Appropriate contents for analytical evaluation : § Contents sufficient § Analytical techniques: robust, simple, effective, quite rapid Answers to regulatory requirements : § HPUS, Ph. Eur. § Consistent with approach taken by Health Authorities in various countries Evaluation on the tincture and mastering of the manufacturing process appropriate for ensuring quality and safety of homeopathic drug products 33

Thank you for your attention Any questions ? 34