Analytical Chemistry ChuanChou River SHEN riverccms ntu edu










- Slides: 25

分析化學 Analytical Chemistry 沈川洲 Chuan-Chou (River) SHEN; river@ccms. ntu. edu. tw TA: 邱瀚毅, r 91224204@ntu. edu. tw; 0912023915 Course description: This course intended is for sophomores the in Dept. of Geosciences. Juniors and seniors are also welcomed. It will approach quantitative analysis first from a classical point of view. Instructor will also review and expand upon the fundamental properties of some modern techniques, which are useful in geochemical analysis. This course emphasizes both theoretical and practical aspects of analytical techniques for geochemistry. Because laboratory experience is the essence of quantitative analysis, a course, “Analytical Chemistry Laboratory”, should be taken together with this course to learn the basis of good laboratory practice. After taking this class, students, with a body of geo-analytical knowledge, will be able to design an experimental procedure to solve real or hypothetical problems. They will also learn how to independently accomplish general chemistry in a geochemical lab.

Literature: Quantitative Chemical Analysis (6 th ed. , 2003) by Daniel C. Harris Fundamentals of Analytical Chemistry by Skoog and West Exploring Chemical Analysis (2 nd ed. , 2001) by Daniel C. Harris Statistics for Analytical Chemistry by Miller and Miller Credits: 2 (+1: experiment) Class hours: 8: 10 -10: 00 pm, Friday Grading: Homework, 30%; Midterm exam, 30%; Final exam, 30%; Class participation, 10%.


Syllabus: Sept Introduction, Units, tools, basic concepts. Oct Error, statistics and excel spreadsheets Calibration methods. Chemical equilibrium. Nov Basic concepts on titrations, Acid-base titration. Introduction to chromatography. Ion exchange chromatography. Nov 28 Midterm exam. Dec Geochemical samples, sampling and preparation. Quality Assurance. Jan Analytical methods for geo-environmental samples. Electrochemistry. Jan 16 Final exam.


Experiments: 1 2 3 4 Balance calibration; dollar statistics. Calibration of volumetric labware. Preparing standard acid and base. Analysis of a mixture of carbonate and bicarbonate. 5 Titration of bases in household drain cleaners. 6 EDTA titration of Ca 2+ and Mg 2+ in natural water 7 Capacity of ion-exchange resins




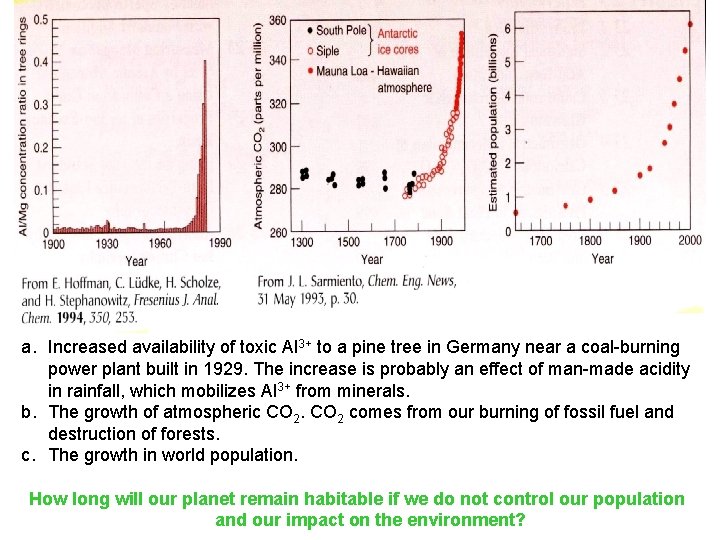
a. Increased availability of toxic Al 3+ to a pine tree in Germany near a coal-burning power plant built in 1929. The increase is probably an effect of man-made acidity in rainfall, which mobilizes Al 3+ from minerals. b. The growth of atmospheric CO 2 comes from our burning of fossil fuel and destruction of forests. c. The growth in world population. How long will our planet remain habitable if we do not control our population and our impact on the environment?




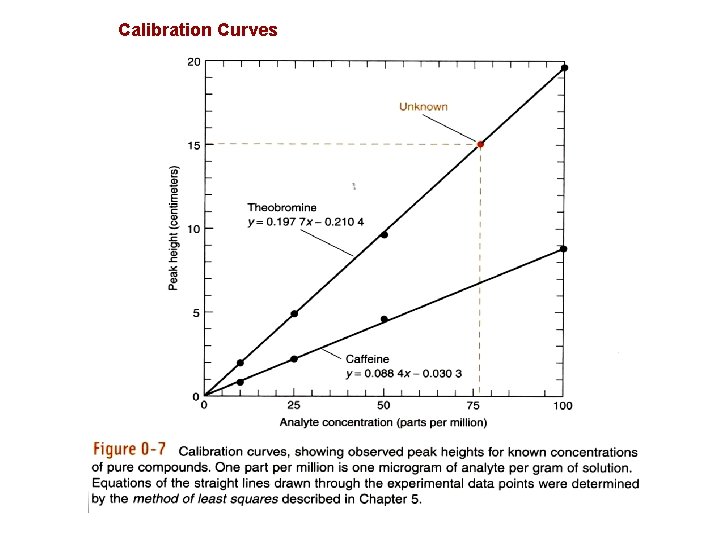
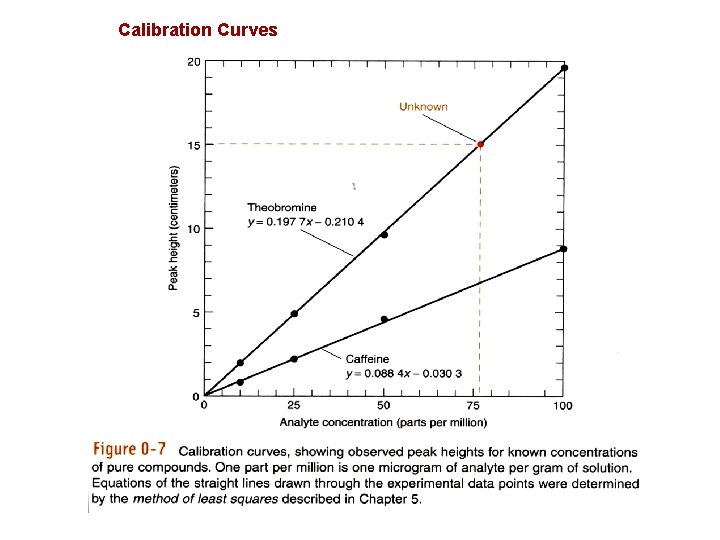
Calibration Curves

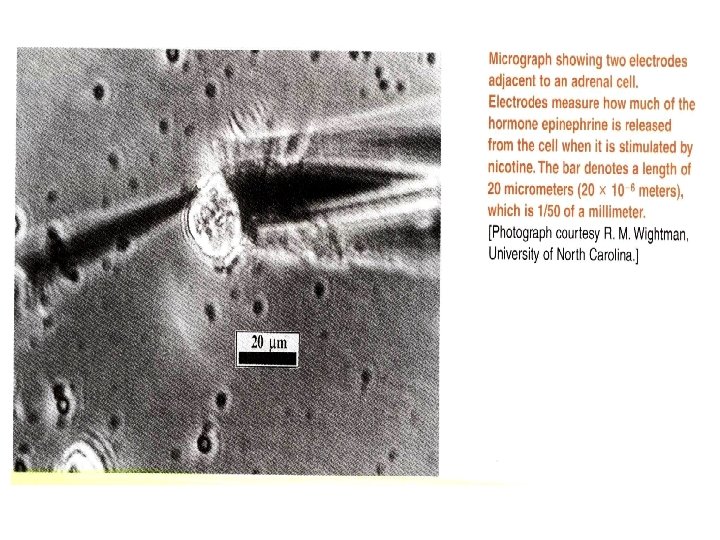
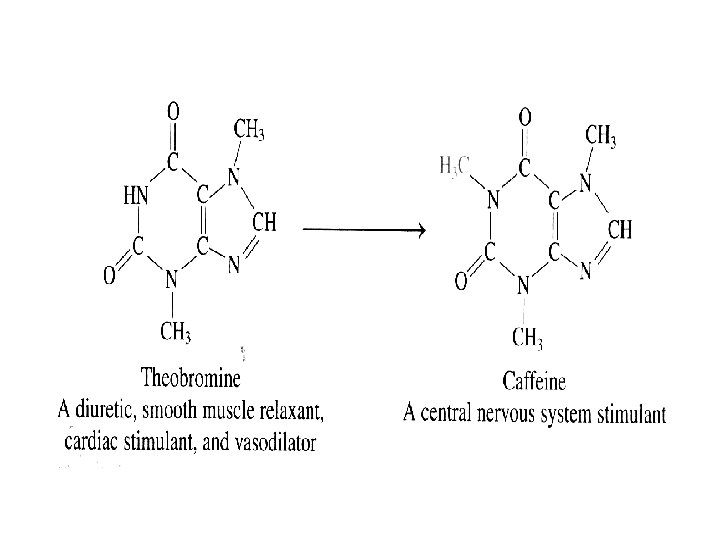
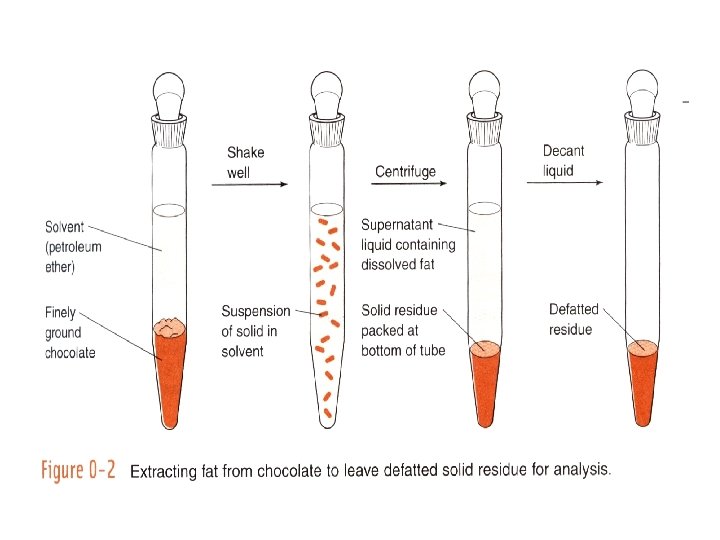
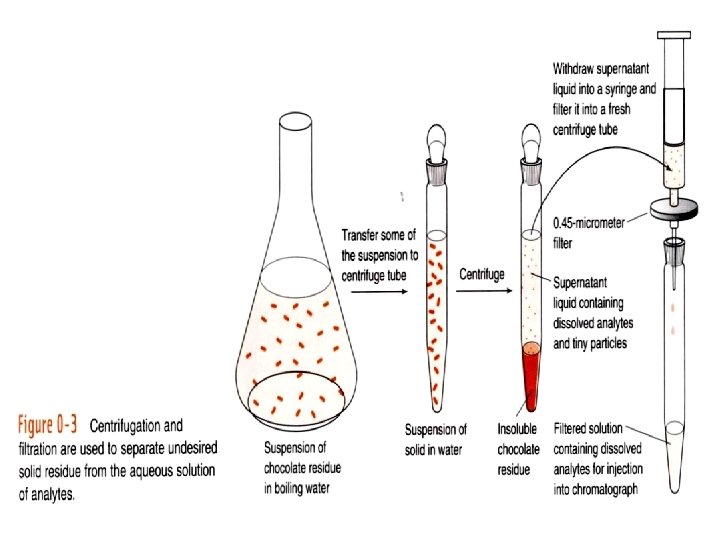
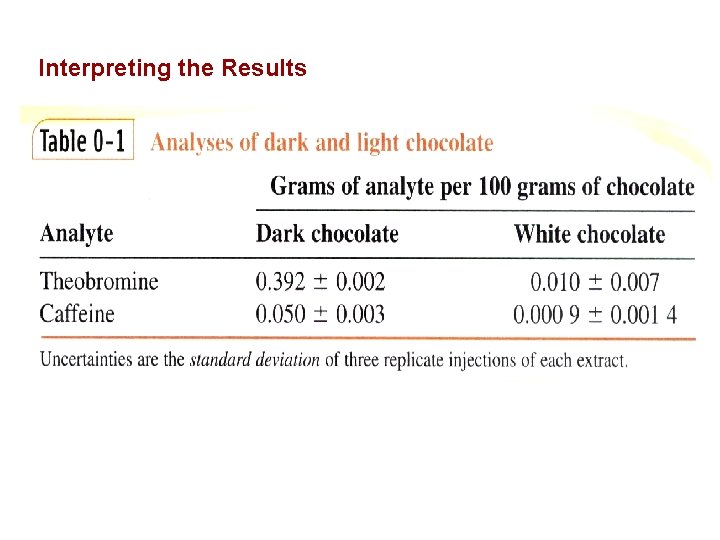
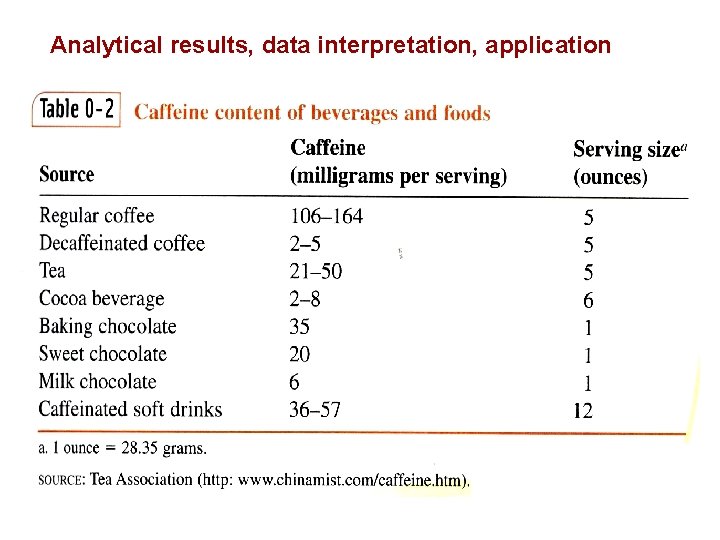
0 -1 The Analytical Chemist’s Job Bates College in Maine, Professor Tom Wenzel Students: Denby and Scott Sampling Homogeneous: same throughout Heterogeneous: differs from region to region, Chocolate with nuts Sample Preparation Substances being measured caffeine and theobromine in this case are called analytes.



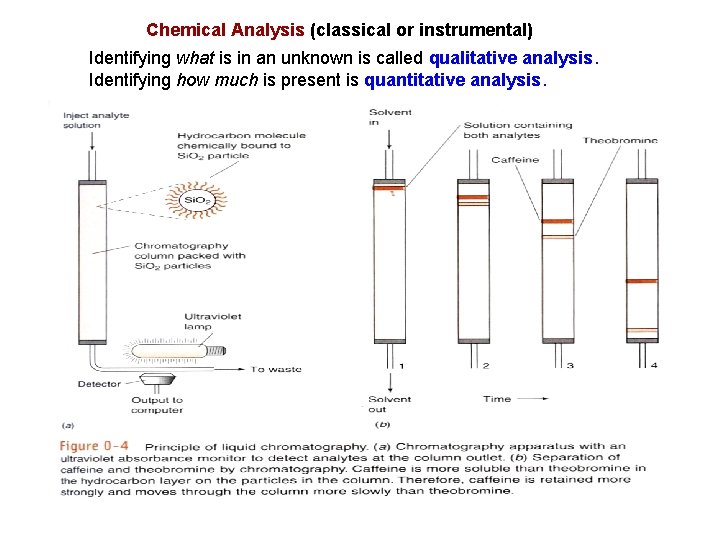
Chemical Analysis (classical or instrumental) Identifying what is in an unknown is called qualitative analysis. Identifying how much is present is quantitative analysis.


Calibration Curves

Interpreting the Results

Analytical results, data interpretation, application

General Steps in a Chemical Analysis 1. Formulating the question 2. Selecting analytical procedures 3. Sampling 4. Sample preparation 5. Analysis 6. Reporting and interpretation 7. Drawing conclusions


 River shen
River shen Indeterminate error
Indeterminate error Ntu.edu.ua:9095
Ntu.edu.ua:9095 Ntu csie
Ntu csie Analysis
Analysis Kesalahan kuantitatif adalah
Kesalahan kuantitatif adalah Jelaskan kegunaan statistika dalam analisis kimia
Jelaskan kegunaan statistika dalam analisis kimia Analytical chemistry apparatus
Analytical chemistry apparatus How to extend analytical measurement range
How to extend analytical measurement range Gaussian curve
Gaussian curve Analytical chemistry definition
Analytical chemistry definition Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry definition and examples
Analytical chemistry definition and examples Q test in analytical chemistry
Q test in analytical chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry chapters
Analytical chemistry chapters Special purpose reagent chemicals
Special purpose reagent chemicals Analytical chemistry
Analytical chemistry Excellence in analytical chemistry
Excellence in analytical chemistry Cengage
Cengage Green river watershed
Green river watershed I am the river and the river is me
I am the river and the river is me Edu.sharif.edu
Edu.sharif.edu Shen, wei-min
Shen, wei-min Haiying shen
Haiying shen