ANALYTICAL CHEMISTRY LECTURE 1 Theoretical fundamentals of analytical














- Slides: 25

ANALYTICAL CHEMISTRY LECTURE 1 • «Theoretical fundamentals of analytical chemistry, analytical chemistry as a science. The subject matter and tasks of analytical chemistry. Chemical analysis and its species. Analytical properties of the substances, analytical reactions and requirements to them. »

The subject matter and tasks of analytical chemistry. • Analytical chemistry is – depending on the point of view – the oldest as well as the youngest branch of chemistry, the science of the transformation of matter. Claiming to be the oldest branch of natural philosophy (as "chemistry" has been described) goes back to the use of the Aristotelian syllogism for argumentation and proof (as shown in his "Analytiken"). Claiming to be the youngest branch became a possibility as "analytics" emerged as an own scientific discipline based on application of modem knowledge theories and information science in chemistry. The emancipation of analytical chemistry from chemistry began with Robert Boyle, continued with the activities of Lavoisier, Berzelius, Wöhler and Liebig, culminated the first time 100 years ago with Wilhem Ostwald (and his work Die wissenschaftlichen Grund-lagen der Analytischen Chemie) and led to its present autonomy as a separate, very complex and highly attractive branch of science, described in its fundamentals in this book.

• The development of analytical chemistry is still continuing at a dramatic rate: consider the dynamic impact of Jan Heyrovsky (Electroanalysis), Richard R. Ernst (NMR), Gerhard Binnig and Heinrich Rohrer (STM/AFM) to name just a few of today's great researchers. As a consequence of the early and continuing interest in this field, a wealth of empirical knowledge, both of fundamental and of practical importance, about the material world in and around us has been compiled.

• Chemistry as a whole has evolved worldwide into a supporting pillar of human culture, industry and trade, providing numerous goods of urgent daily need for humankind, such as food, clothing, shelter, pharmaceuticals and materials essential for medical use, transport or communication. Purely empirical at first, and mainly a branch of medicine, today's chemistry is a modem experimental science under-pinned by physico-chemical and mathematical laws and has itself diversified into organic chemistry, inorganic chemistry, biochemistry, food chemistry, chemical technologies, physical chemistry and lately analytical chemistry. • Analytical chemistry is an in-between-science using and depending on the laws of chemistry, physics, mathematics, information science and biology. Its aim is to decipher the information hidden in the sample under investigation, not to change this intrinsic information, hence to tell the truth about the composition of the material.

Applications • Analytical chemistry has applications including in forensics, bioanalysis, clinical analysis, environmental analysis, and materials analysis. Analytical chemistry research is largely driven by performance (sensitivity, detection limit, selectivity, robustness, dynamic range, linear range, accuracy, precision, and speed), and cost (purchase, operation, training, time, and space). Among the main branches of contemporary analytical atomic spectrometry, the most widespread and universal are optical and mass spectrometry. • In the direct elemental analysis of solid samples, the new leaders are laser-induced breakdown and laser ablation mass spectrometry, and the related techniques with transfer of the laser ablation products into inductively coupled plasma. Advances in design of diode lasers and optical parametric oscillators promote developments in fluorescence and ionization spectrometry and also in absorption techniques where uses of optical cavities for increased effective absorption pathlength are expected to expand. The use of plasma- and laser-based methods is increasing. An interest towards absolute (standardless) analysis has revived, particularly in emission spectrometry.

• Great effort is being put in shrinking the analysis techniques to chip size. Although there are few examples of such systems competitive with traditional analysis techniques, potential advantages include size/portability, speed, and cost. (micro total analysis system (µTAS) or lab-on-a-chip). Microscale chemistry reduces the amounts of chemicals used. • Many developments improve the analysis of biological systems. Examples of rapidly expanding fields in this area are genomics, DNA sequencing and related research in genetic fingerprinting and DNA microarray; proteomics, the analysis of protein concentrations and modifications, especially in response to various stressors, at various developmental stages, or in various parts of the body, metabolomics, which deals with metabolites; transcriptomics, including m. RNA and associated fields; lipidomics - lipids and its associated fields; peptidomics - peptides and its associated fields; and metalomics, dealing with metal concentrations and especially with their binding to proteins and other molecules. • Analytical chemistry has played critical roles in the understanding of basic science to a variety of practical applications, such as biomedical applications, environmental monitoring, quality control of industrial manufacturing, forensic science and so on.

• The recent developments of computer automation and information technologies have extended analytical chemistry into a number of new biological fields. For example, automated DNA sequencing machines were the basis to complete human genome projects leading to the birth of genomics. Protein identification and peptide sequencing by mass spectrometry opened a new field of proteomics. • Analytical chemistry has been an indispensable area in the development of nanotechnology. Surface characterization instruments, electron microscopes and scanning probe microscopes enables scientists to visualize atomic structures with chemical characterizations.

The history • Analytical chemistry has been important since the early days of chemistry, providing methods for determining which elements and chemicals are present in the object in question. During this period significant contributions to analytical chemistry include the development of systematic elemental analysis by Justus von Liebig and systematized organic analysis based on the specific reactions of functional groups. • The first instrumental analysis was flame emissive spectrometry developed by Robert Bunsen and Gustav Kirchhoff who discovered rubidium (Rb) and caesium (Cs) in 1860. • Most of the major developments in analytical chemistry take place after 1900. During this period instrumental analysis becomes progressively dominant in the field. In particular many of the basic spectroscopic and spectrometric techniques were discovered in the early 20 th century and refined in the late 20 th century.

• The separation sciences follow a similar time line of development and also become increasingly transformed into high performance instruments. [5] In the 1970 s many of these techniques began to be used together as hybrid techniques to achieve a complete characterization of samples. • Starting in approximately the 1970 s into the present day analytical chemistry has progressively become more inclusive of biological questions (bioanalytical chemistry), whereas it had previously been largely focused on inorganic or small organic molecules. Lasers have been increasingly used in chemistry as probes and even to initiate and influence a wide variety of reactions. The late 20 th century also saw an expansion of the application of analytical chemistry from somewhat academic chemical questions to forensic, environmental, industrial and medical questions, such as in histology. • Modern analytical chemistry is dominated by instrumental analysis. Many analytical chemists focus on a single type of instrument. Academics tend to either focus on new applications and discoveries or on new methods of analysis. The discovery of a chemical present in blood that increases the risk of cancer would be a discovery that an analytical chemist might be involved in. An effort to develop a new method might involve the use of a tunable laser to increase the specificity and sensitivity of a spectrometric method. Many methods, once developed, are kept purposely static so that data can be compared over long periods of time. This is particularly true in industrial quality assurance (QA), forensic and environmental applications. Analytical chemistry plays an increasingly important role in the pharmaceutical industry where, aside from QA, it is used in discovery of new drug candidates and in clinical applications where understanding the interactions between the drug and the patient are critical.

• Analytical chemistry - one of fundamental chemical disciplines, that mortgages bases for the further study of profile disciplines. • Analytical chemistry is the science about the methods of study of composition of substance. • The course of analytical chemistry consists of three parts: • - it is a qualitative analysis; • - it is a quantitative analysis; • - are instrumental methods of analysis.

• A qualitative analysis is a process of authentication of substance, allowing to set from what chemical elements there is the investigated test, what ions, functional groups or molecules included in it �s composition. A qualitative analysis determines the presence or absence of a particular compound, but not the mass or concentration. By definition, qualitative analyses do not measure quantity. • Depending on composition of the investigated mixture distinguish: - an analysis of inorganics, including determination of cations and anions; - an analysis of organic substances, that is subdivided into an element analysis - discovery and determination of elements, and functional analysis - analysis of functional groups consisting of a few chemical elements and being certain characteristics; - a molecular analysis is an analysis of separate compounds.

• A primary objective of qualitative analysis is a discovery in the investigated test of separate cations, anions, functional groups, molecules or elements that is included in it as composition, with the use of chemical, physical and physico - chemical methods of analysis. • In the chemical methods of quality analysis use characteristic quality analytical reactions for that certain external displays are inherent : education in solutions of the coloured fallouts, discoloration of solution, selection of gaseous foods with a smell or odourless. • A substance, that is used for realization of quality analytical reaction is a reagent.

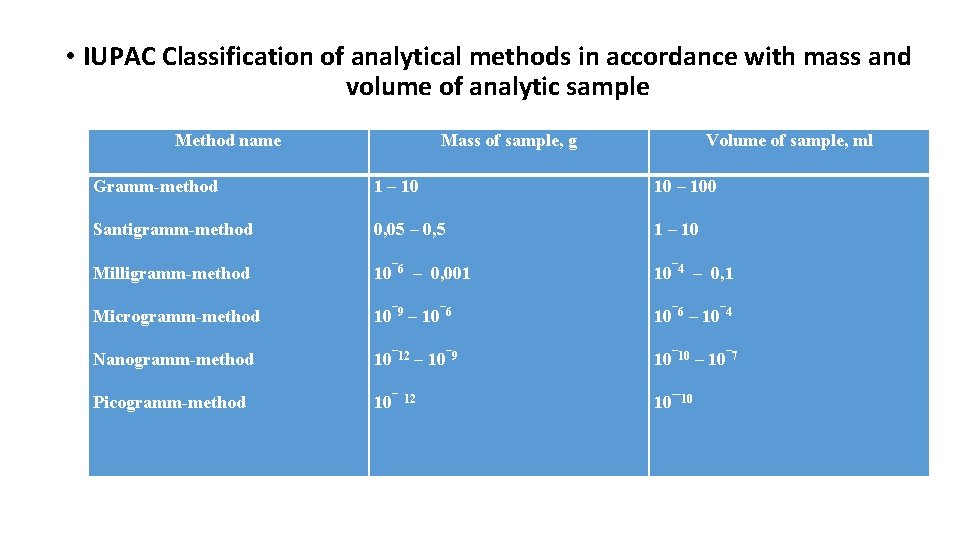
• IUPAC Classification of analytical methods in accordance with mass and volume of analytic sample Method name Mass of sample, g Volume of sample, ml Gramm-method 1 – 10 10 – 100 Santigramm-method 0, 05 – 0, 5 1 – 10 Milligramm-method 10¯ 6 – 0, 001 10¯ 4 – 0, 1 Microgramm-method 10¯ 9 – 10¯ 6 – 10¯ 4 Nanogramm-method 10¯ 12 – 10¯ 9 10¯ 10 – 10¯ 7 Picogramm-method 10¯ 10 12

• Depending on a size sample drawn a for an analysis methods divide into: macro-, semimicro-, and ultramicromethods of quality analysis. • Macroanalysis: a 0, 5 -1, 0 g of substance or 20 -50 mls of solution; • Microanalysis: a 0, 01 - 0, 001 g of substance or 0, 05 - 0, 5 mls of solution. • Semimicroanalysis occupies an intermediate place between macro- and by micromethods. In this case for an analysis use a 0. 01 - 0. 1 g of dry substance or 0, 5 - 5, 0 mls of solution.

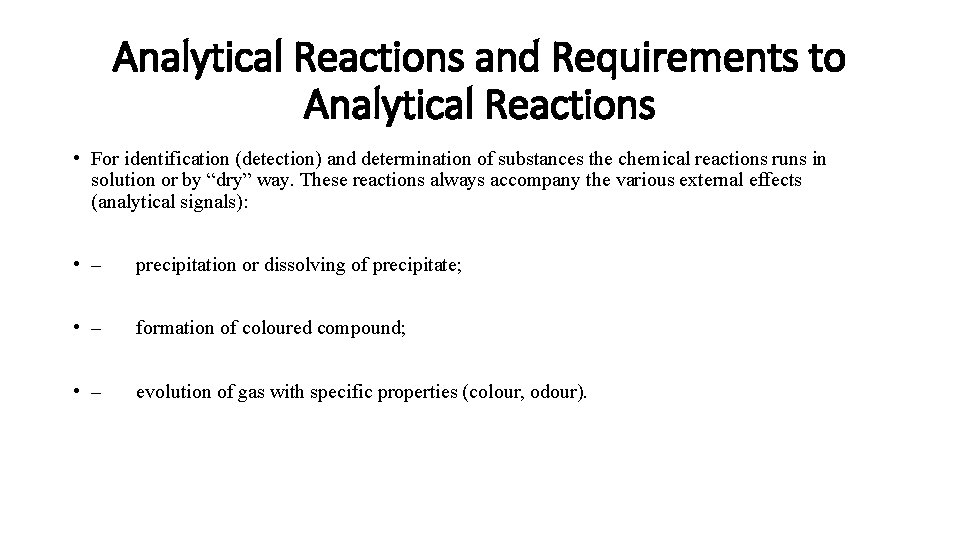
Analytical Reactions and Requirements to Analytical Reactions • For identification (detection) and determination of substances the chemical reactions runs in solution or by “dry” way. These reactions always accompany the various external effects (analytical signals): • – precipitation or dissolving of precipitate; • – formation of coloured compound; • – evolution of gas with specific properties (colour, odour).

“Dry” way testing (without dissolving of sample) can be make by: 1) pyrochemical methods: – flame test (colouring of gas torch flame), – making a glass (alloys with Na 2 CO 3, K 2 CO 3, Na 2 B 4 O 7, Na(NH 4)2 PO 4), – tempering; 2) crush (rub) sample to powder with analytical reagent; 3) microcrystalloscopic analysis – produce (receive) the specific crystals with analytical reagent and watching its with microscope (forms of crystals); 4) analysis in drops on filter paper – reaction between analysed substance and analytical reagent run on filter paper with some drops (1 -2) of solutions – arise a coloured spots.

Requirements (demands) to analytical reactions: • 1) reaction must run quickly, in practice – immediately; • 2) reaction must accompanied with accordance (special) analytical effect; • 3) reaction must be irreversible – run in one way (in one side); • 4) reaction must have high specificity and have high sensitivity.

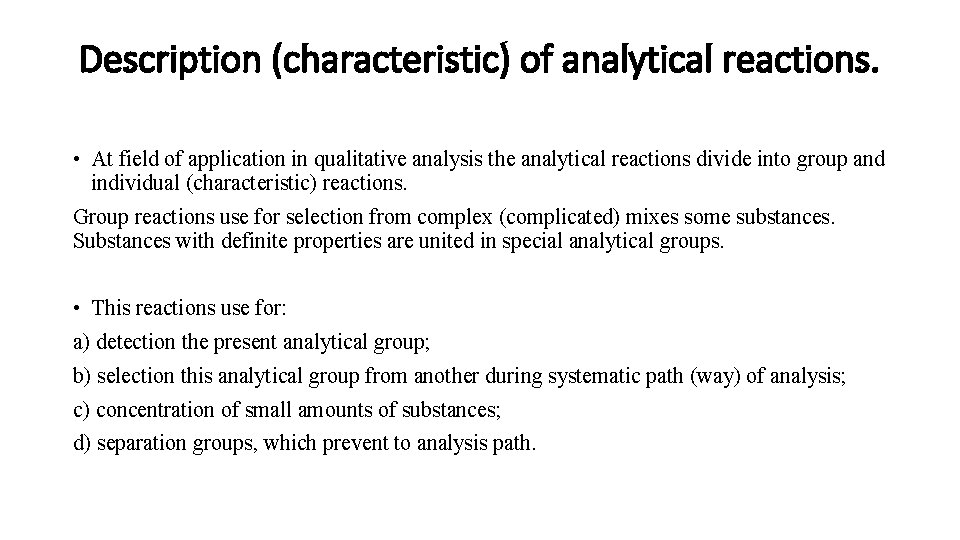
Description (characteristic) of analytical reactions. • At field of application in qualitative analysis the analytical reactions divide into group and individual (characteristic) reactions. Group reactions use for selection from complex (complicated) mixes some substances. Substances with definite properties are united in special analytical groups. • This reactions use for: a) detection the present analytical group; b) selection this analytical group from another during systematic path (way) of analysis; c) concentration of small amounts of substances; d) separation groups, which prevent to analysis path.

Characteristic reactions named analytical reactions that have the individual substance nature. These reactions distinguish to selectivity. Selective reactions give identical or alike analytical effects with small (little) number of ions (2 -5). Extreme form of selectivity is specificity. Specific reaction gives an analytical effect only with one individual substance. For examples(specific reactions): – iodine with starch – complex compound blue (navy) colour; – Fe+3 with K 4[Fe(CN)6] – complex compound blue (navy) colour.

One of important requirements to the analytical reactions - from high sensitiveness (selectivity). The sensitiveness (selectivity) of reaction is name the smallest concentration of ions, that it can find out by means of this reaction at certain terms. Analytical reactions that are used in a qualitative analysis divide into specific and heterospecific (selective). A specific reaction on certain ions is named a reaction that allows to define these ions in the conditions of experience in presence of other ions without their preliminary selection. For example, a specific reaction on the ions of ammonium is their co-operating with solutions of lyes at heating. An ammonia that is distinguished is determined on a smell or on discoloration of red litmus paper. A reaction that gives an analytical effect in presence a limit number of ions is named heterospecific or selective.

• There a bit specific reactions in a quality analysis, therefore the selective use more often, that require application of the special methods of removal of mixing influence of other substances being in a test. It is arrived at by dividing of the system into component parts ( more often than sinking solution), that at volume ions interfering with finding out the determined ions were in other phase In accordance with it distinguish two methods of quality analysis : • - shot; • - systematic. In a fractional analysis composition of analysable test is determined by specific in certain terms reactions that allow to find out the investigated ions in presence of other ions. Implementation of fractional analysis is conducted in two stages: in the beginning by means of different reactions remove influence of mixing components, and then find out the determined ions.

• Systematic motion of analysis consists of that difficult mixture of ions at first is divided by means of the so-called group reagents on a few separate groups. Then within the limits of each of them find out separate ions certain characteristic reactions. A group reagent on a certain analytical group specifically reacts with the ions of this group. Analytical reactions allow us to determine same quantity (amount) of substance.

• Sensitivity of analytical reaction is the least amount (quantity) of substance, which can be detected with the reagent in one drop of solution (1 mm 3). • The sensitivity express to next correlated values: Limit of detection = Detected limit (m) – the least amount of substance, which present in analysed solution and which detect with the reagent. Calculate in mg. 1 mg = 0, 000001 g. Limit of concentration = Minimal concentration (Cmin) – the least concentration of solution with still can be detected an analysed substance in definite (one drop) volume. Limit of dilution (W = 1/Cmin) – quantity (ml) of water solution, containing 1 g of the analysed substance, which detect with definite reaction (reagent).

Thus, the sensitivity of analytical reaction is as more as limit of detection and limit of concentration are less. These parameters are connected such: m = Cmin·Vmin· 106 = Vmin· 106 / W

• The natural sciences begin with observation, and this usually involves numerical measurements of quantities such as length, volume, density, and temperature. Most of these quantities have units of some kind associated with them, and these units must be retained when you use them in calculations. Measuring units can be defined in terms of a very small number of fundamental ones that, through "dimensional analysis", provide insight into their derivation and meaning, and must be understood when converting between different unit systems.