All living systems require constant input of free





- Slides: 32

All living systems require constant input of free energy. Metabolism and Energy AP Biology

The First Law of Thermodynamics Energy cannot be created or destroyed, only transformed. Living systems need to continually acquire and transform energy in order to remain alive. “Free energy”: The energy available in a system to do work. AP Biology

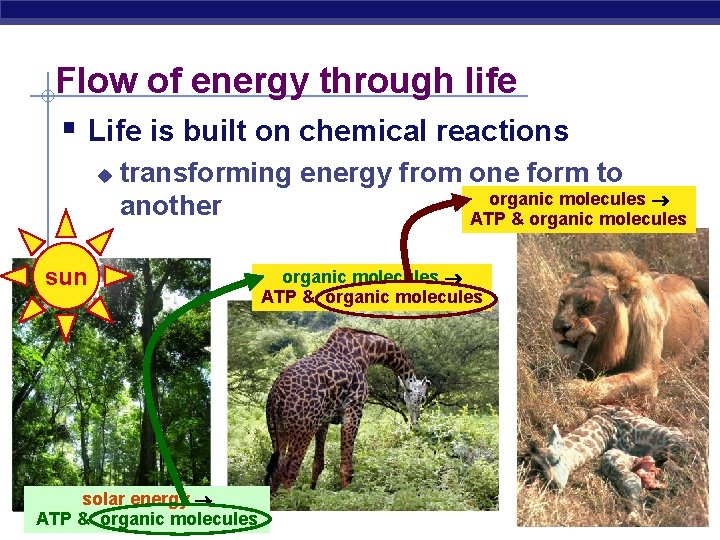
Flow of energy through life § Life is built on chemical reactions u transforming energy from one form to organic molecules another ATP & organic molecules sun solar energy AP Biology ATP & organic molecules

The 2 nd Law of Thermodynamics Every time energy is transformed, the entropy (“disorder”) of the universe increases. In order to increase/ maintain their internal order, living systems must process more ordered forms of matter in to less ordered ones AP Biology

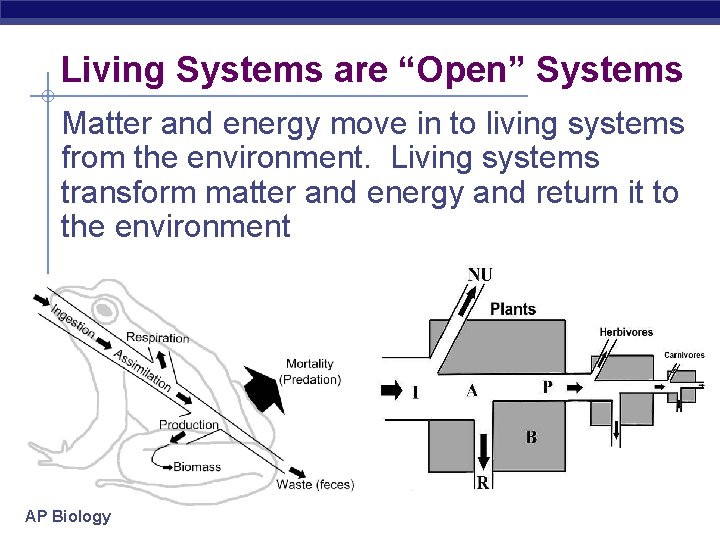
Living Systems are “Open” Systems Matter and energy move in to living systems from the environment. Living systems transform matter and energy and return it to the environment AP Biology

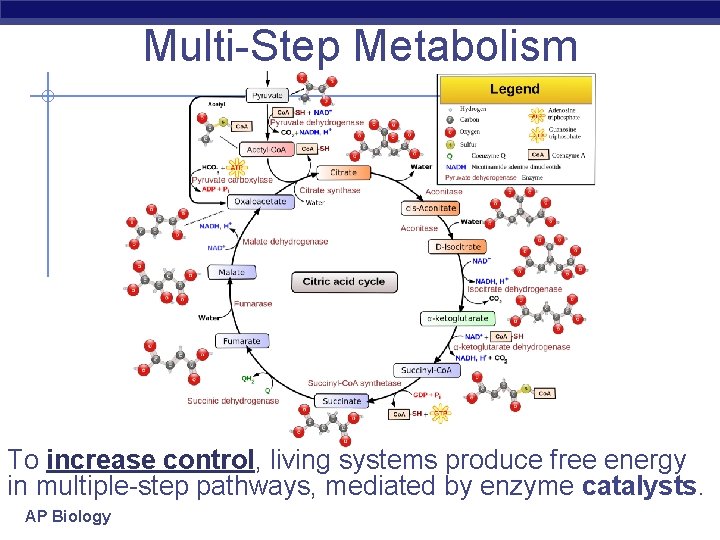
Multi-Step Metabolism To increase control, living systems produce free energy in multiple-step pathways, mediated by enzyme catalysts. AP Biology

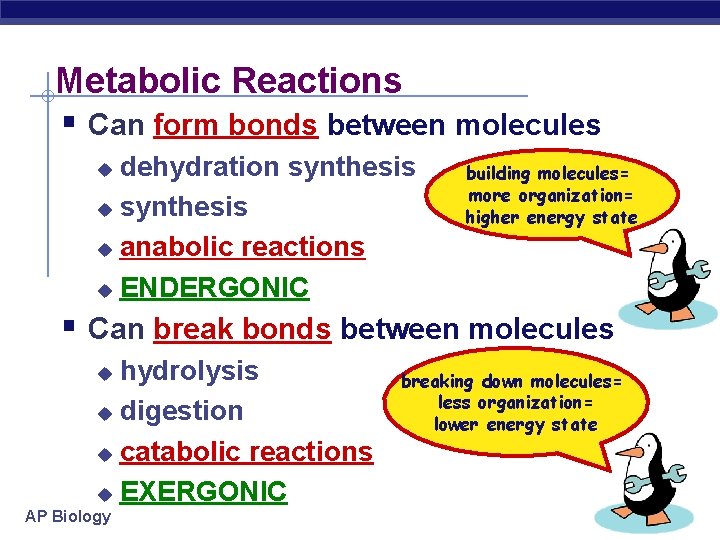
Metabolic Reactions § Can form bonds between molecules dehydration synthesis u anabolic reactions u ENDERGONIC u building molecules= more organization= higher energy state § Can break bonds between molecules hydrolysis u digestion u catabolic reactions u EXERGONIC u AP Biology breaking down molecules= less organization= lower energy state

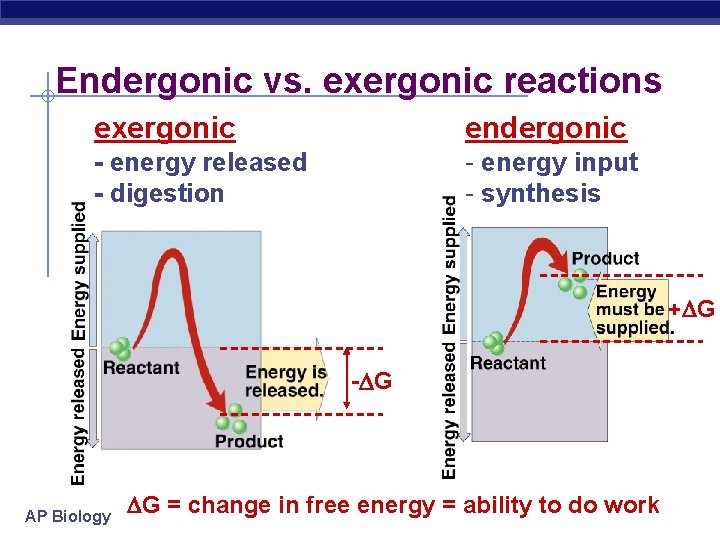
Endergonic vs. exergonic reactions exergonic endergonic - energy released - digestion - energy input - synthesis + G - G AP Biology G = change in free energy = ability to do work

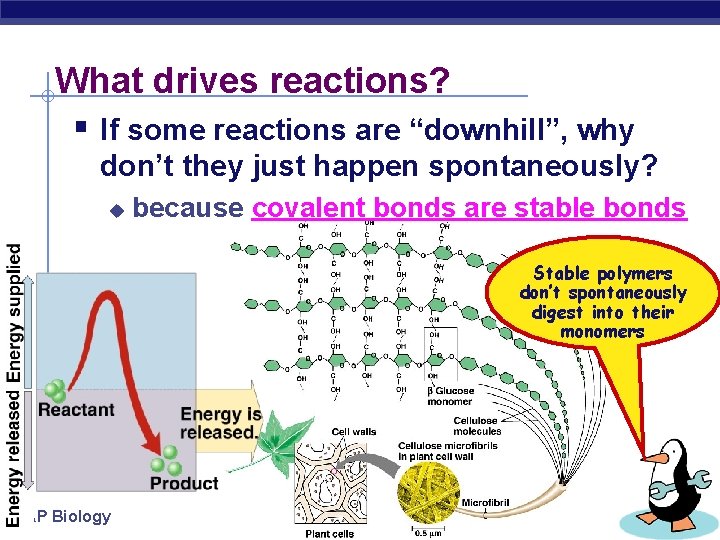
What drives reactions? § If some reactions are “downhill”, why don’t they just happen spontaneously? u because covalent bonds are stable bonds Stable polymers don’t spontaneously digest into their monomers AP Biology

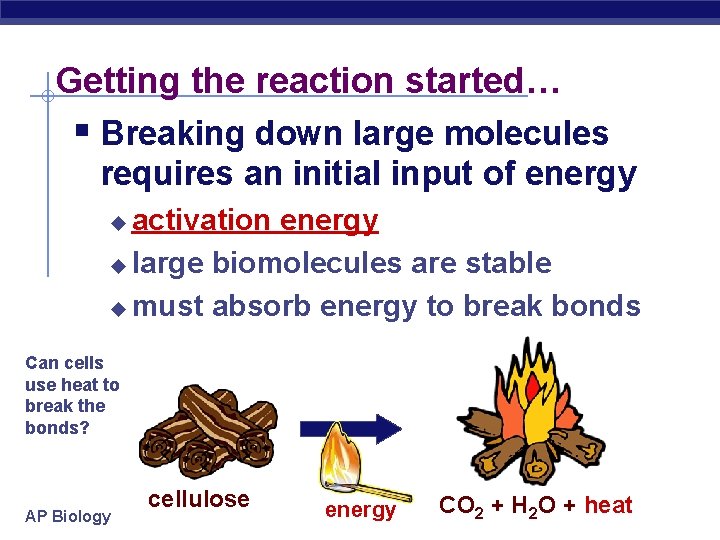
Getting the reaction started… § Breaking down large molecules requires an initial input of energy activation energy u large biomolecules are stable u must absorb energy to break bonds u Can cells use heat to break the bonds? AP Biology cellulose energy CO 2 + H 2 O + heat

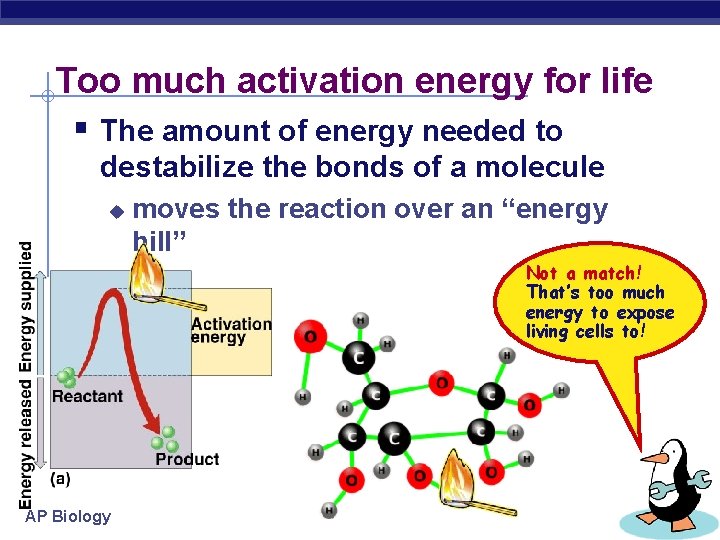
Too much activation energy for life § The amount of energy needed to destabilize the bonds of a molecule u moves the reaction over an “energy hill” Not a match! That’s too much energy to expose living cells to! AP Biology

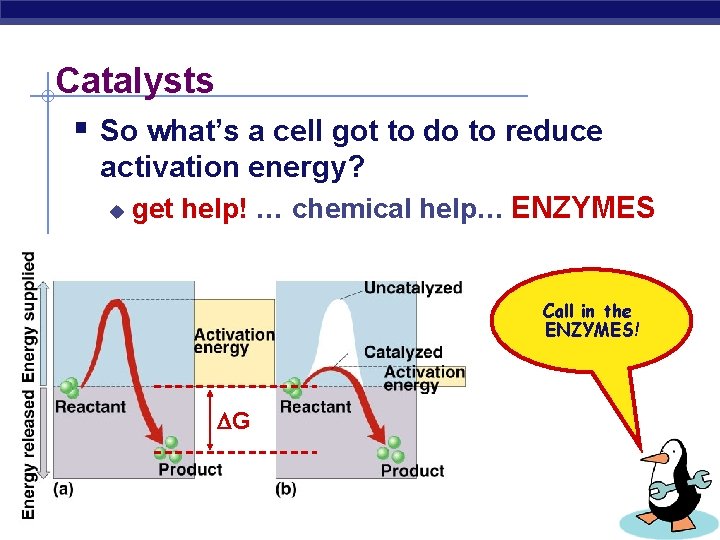
Catalysts § So what’s a cell got to do to reduce activation energy? u get help! … chemical help… ENZYMES Call in the ENZYMES! G AP Biology

Energy needs of life § Organisms are endergonic systems u What do we need energy for? § § § AP Biology synthesis (biomolecules) reproduction active transport movement temperature regulation 2005 -2006

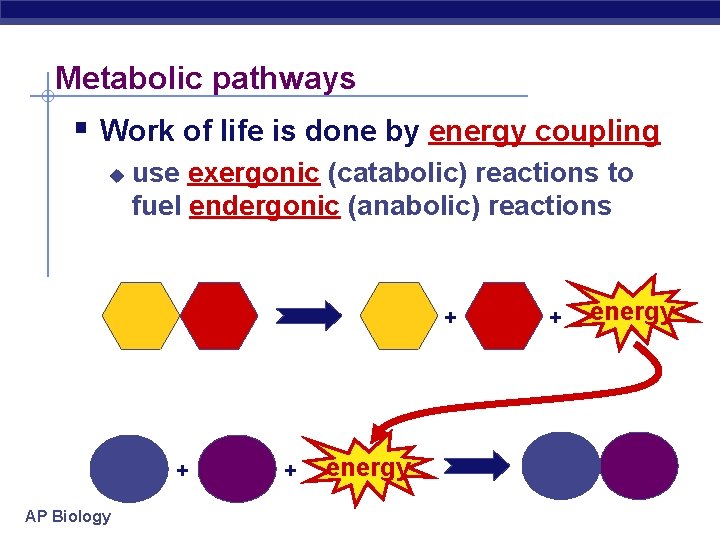
Metabolic pathways § Work of life is done by energy coupling u use exergonic (catabolic) reactions to fuel endergonic (anabolic) reactions + + AP Biology + energy

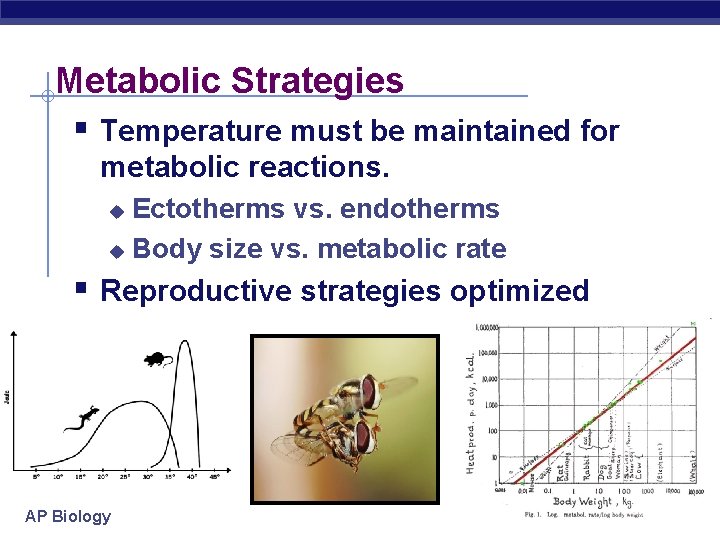
Metabolic Strategies § Temperature must be maintained for metabolic reactions. Ectotherms vs. endotherms u Body size vs. metabolic rate u § Reproductive strategies optimized AP Biology

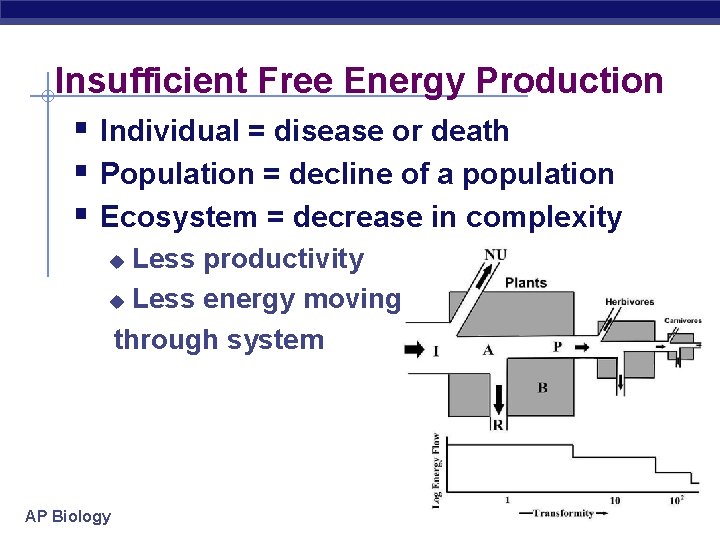
Insufficient Free Energy Production § Individual = disease or death § Population = decline of a population § Ecosystem = decrease in complexity Less productivity u Less energy moving through system u AP Biology

Living economy § Fueling the body’s economy u eat high energy organic molecules § food = carbohydrates, lipids, proteins, nucleic acids u break them down § catabolism = digest u capture released energy in a form the cell can use § Uses an energy currency u u AP Biology a way to pass energy around need a short term energy storage molecule Whoa! Hot stuff! ATP

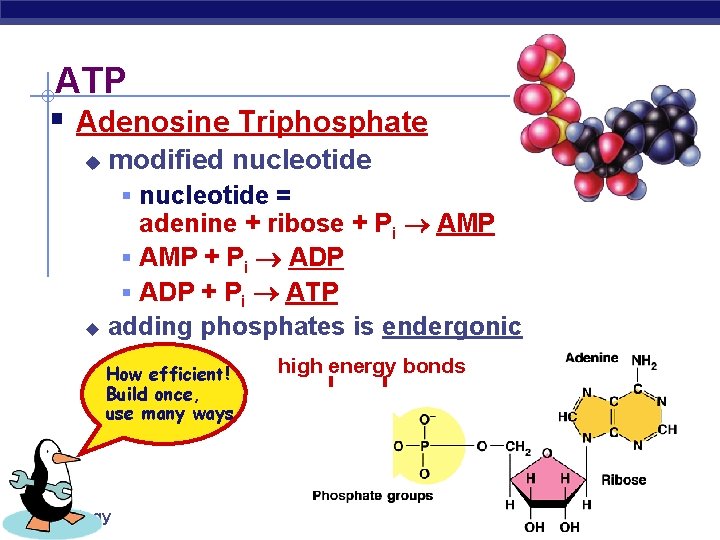
ATP § Adenosine Triphosphate u modified nucleotide § nucleotide = adenine + ribose + Pi AMP § AMP + Pi ADP § ADP + Pi ATP u adding phosphates is endergonic How efficient! Build once, use many ways AP Biology high energy bonds

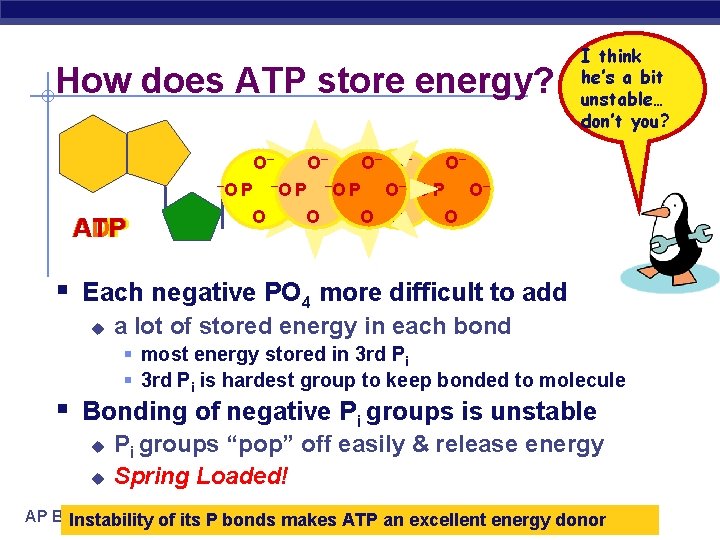
How does ATP store energy? O– ADP AMP ATP I think he’s a bit unstable… don’t you? O– O– O– –O P –O–P–O PO– –O–P O– O O O– § Each negative PO 4 more difficult to add u a lot of stored energy in each bond § most energy stored in 3 rd Pi § 3 rd Pi is hardest group to keep bonded to molecule § Bonding of negative Pi groups is unstable u u Pi groups “pop” off easily & release energy Spring Loaded! AP Biology Instability of its P bonds makes ATP an excellent energy donor

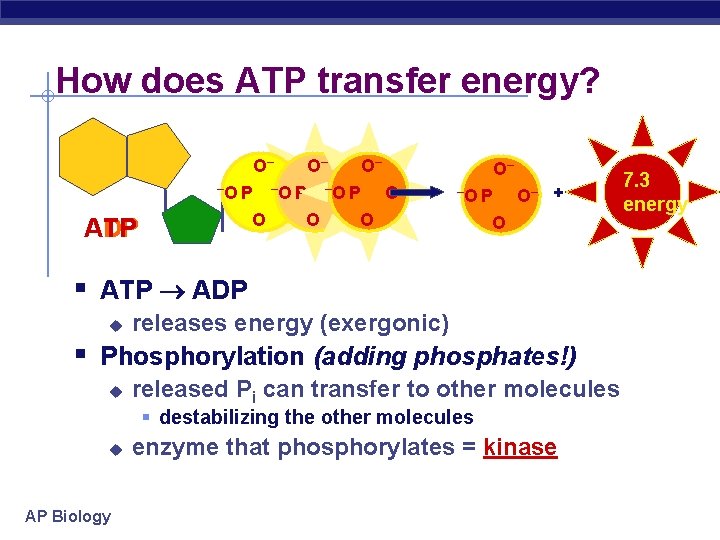
How does ATP transfer energy? O– –O P O ATP ADP O– –O–P O– O– –O P O O– + O § ATP ADP u releases energy (exergonic) § Phosphorylation (adding phosphates!) u released Pi can transfer to other molecules § destabilizing the other molecules u AP Biology enzyme that phosphorylates = kinase 7. 3 energy

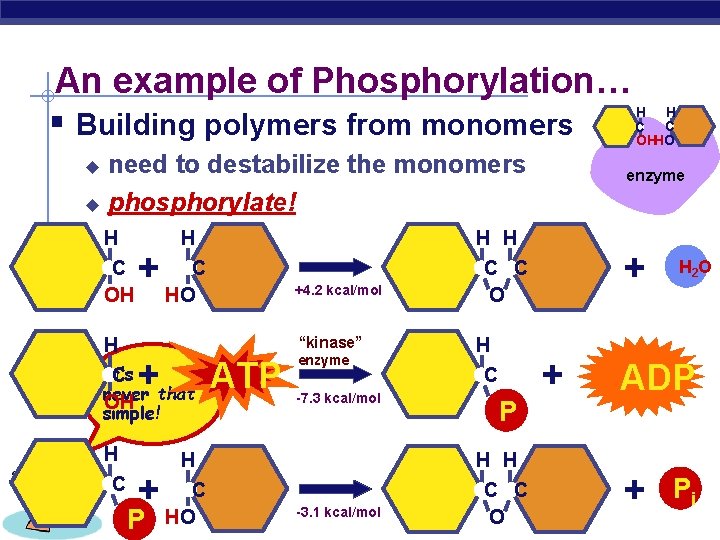
An example of Phosphorylation… § Building polymers from monomers need to destabilize the monomers u phosphorylate! u H C OH H + H C AP Biology +4. 2 kcal/mol “kinase” It’s C never that OH simple! + P ATP enzyme -7. 3 kcal/mol H C HO enzyme H H C HO C P H H -3. 1 kcal/mol + C C O H H C C OHHO + H 2 O ADP + Pi

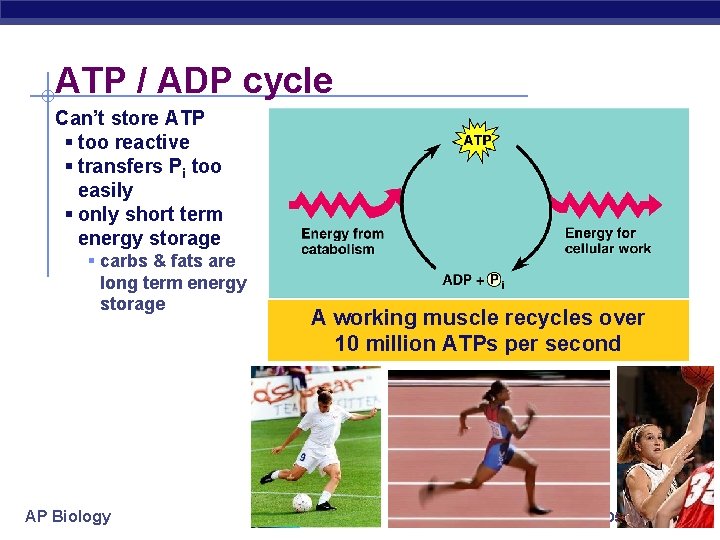
ATP / ADP cycle Can’t store ATP § too reactive § transfers Pi too easily § only short term energy storage § carbs & fats are long term energy storage AP Biology A working muscle recycles over 10 million ATPs per second 2005 -2006

What’s the point? § Cells spend a lot of time making ATP! “WHY? ” For chemical, mechanical, and transport work Make ATP! That’s all I do all day. And no one even notices! AP Biology

3. 1: All living systems require constant input of free energy. 2. MATH SKILLS: GIBBS FREE ENERGY AP Biology

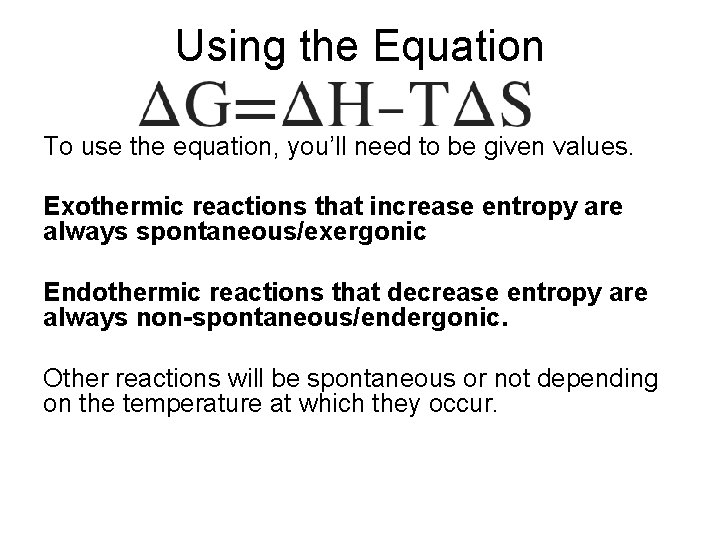
What You Have To Do Be able to use and interpret the Gibbs Free Energy Equation to determine if a particular process will occur spontaneously or non-spontaneously. ΔG= change in free energy (- = exergonic, + = endergonic) ΔH= change in enthalpy for the reaction (- = exothermic, + = endothermic) T = kelvin temperature ΔS = change in entropy (+ = entropy increase, - = entropy decrease)

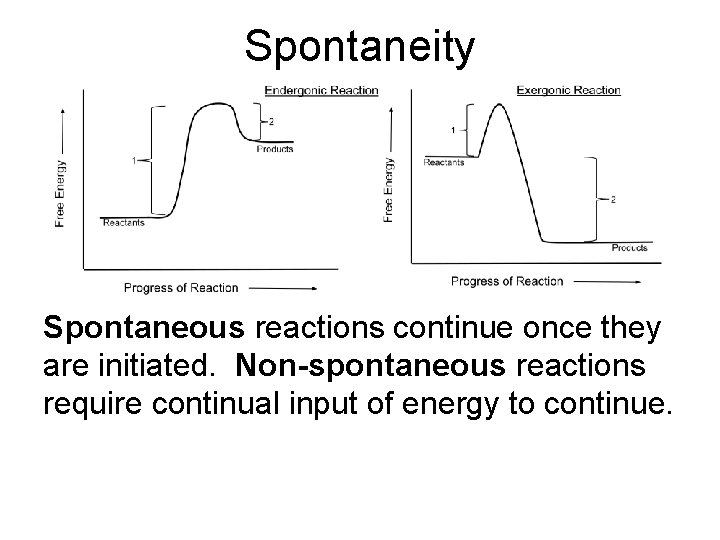
Spontaneity Spontaneous reactions continue once they are initiated. Non-spontaneous reactions require continual input of energy to continue.

Using the Equation To use the equation, you’ll need to be given values. Exothermic reactions that increase entropy are always spontaneous/exergonic Endothermic reactions that decrease entropy are always non-spontaneous/endergonic. Other reactions will be spontaneous or not depending on the temperature at which they occur.

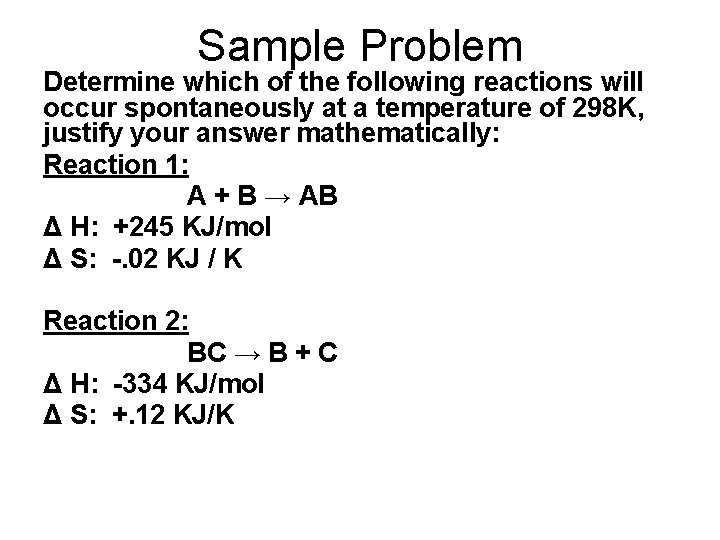
Sample Problem Determine which of the following reactions will occur spontaneously at a temperature of 298 K, justify your answer mathematically: Reaction 1: A + B → AB Δ H: +245 KJ/mol Δ S: -. 02 KJ / K Reaction 2: BC → B + C Δ H: -334 KJ/mol Δ S: +. 12 KJ/K

3. 1: All living systems require constant input of free energy. 4. MATH SKILLS: COEFFICIENT Q 10

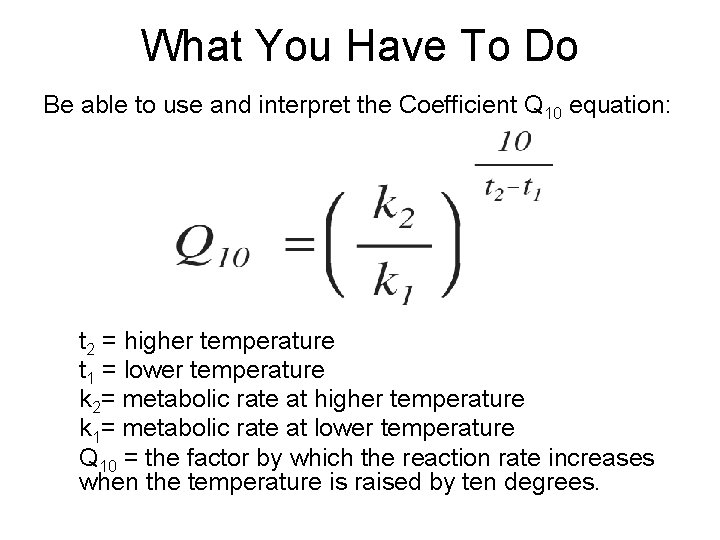
What You Have To Do Be able to use and interpret the Coefficient Q 10 equation: t 2 = higher temperature t 1 = lower temperature k 2= metabolic rate at higher temperature k 1= metabolic rate at lower temperature Q 10 = the factor by which the reaction rate increases when the temperature is raised by ten degrees.

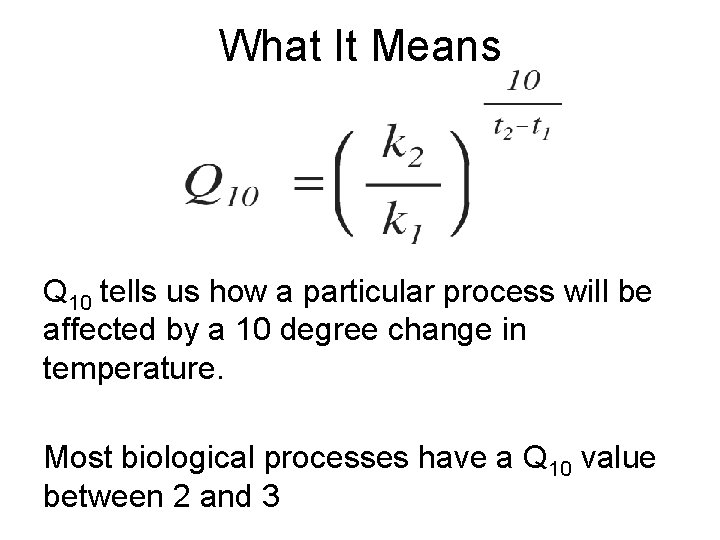
What It Means Q 10 tells us how a particular process will be affected by a 10 degree change in temperature. Most biological processes have a Q 10 value between 2 and 3

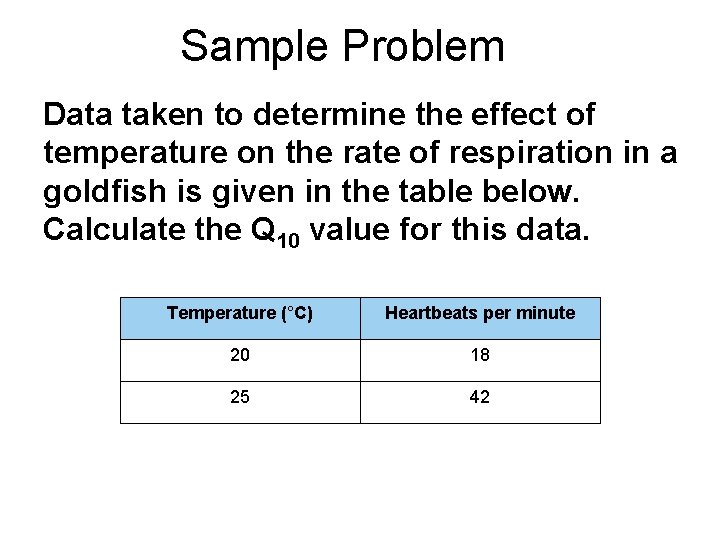
Sample Problem Data taken to determine the effect of temperature on the rate of respiration in a goldfish is given in the table below. Calculate the Q 10 value for this data. Temperature (°C) Heartbeats per minute 20 18 25 42
 What is the smallest living unit
What is the smallest living unit Constant pointer and pointer to constant
Constant pointer and pointer to constant What is pointer to pointer in c
What is pointer to pointer in c 9 pointers
9 pointers Formation constant vs equilibrium constant
Formation constant vs equilibrium constant Equilibrium occurs when
Equilibrium occurs when Big k vs little k chemistry
Big k vs little k chemistry Complex ion formation and solubility
Complex ion formation and solubility Constant to pointer in c
Constant to pointer in c Constant pointer and pointer to constant
Constant pointer and pointer to constant Ksp values table pdf
Ksp values table pdf Specific cake resistance definition
Specific cake resistance definition R constant
R constant Opposite rays
Opposite rays Venn diagram of living things and nonliving things
Venn diagram of living things and nonliving things Moss living or nonliving
Moss living or nonliving Living non living dead
Living non living dead Free fall constant acceleration
Free fall constant acceleration Where do we get a constant supply of free energy
Where do we get a constant supply of free energy Yang bukan merupakan peripheral output adalah
Yang bukan merupakan peripheral output adalah Finely tuned input
Finely tuned input Naegleria sp
Naegleria sp Turbelaria
Turbelaria Equilibrium formula
Equilibrium formula All matter is in constant
All matter is in constant Intro to matter
Intro to matter Seven life processes
Seven life processes Mother of all living
Mother of all living 8 levels of classification for all living things
8 levels of classification for all living things Cells group together to form
Cells group together to form Life cycle of all living things
Life cycle of all living things What are the 5 basic needs of all living things
What are the 5 basic needs of all living things Are all living things based on the metric system
Are all living things based on the metric system