AIR POLLUTION Dr Wesam Al Madhoun What is
















































- Slides: 63

AIR POLLUTION Dr. Wesam Al Madhoun

What is air pollution? • The presence of any substances in the atmosphere in quantities which are or may be harmful or injurious to human health, welfare, animal or plant life, or property or unreasonably interfere with the enjoyment of life or property. 2

Outdoor Air Pollution

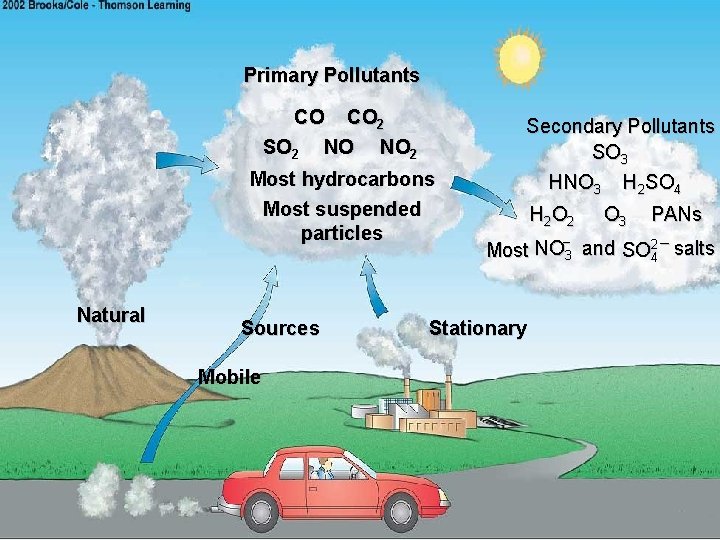
Primary Pollutants CO CO 2 SO 2 NO NO 2 Most hydrocarbons Most suspended particles Natural Sources Mobile Secondary Pollutants SO 3 HNO 3 H 2 SO 4 H 2 O 3 PANs – Most NO 3 and SO 24 – salts Stationary

Primary vs. Secondary Pollutants • Primary- put directly into air from polluting source. • Secondary- when primary combines with other substances in air and creates something more hazardous (acid rain, smog) • Sun often provides energy.

Major Sources of Primary Pollutants Stationary Sources • Combustion of fuels for power and heat – Power Plants • Other burning such as wood & crop burning or forest fires • Industrial/ commercial processes • Solvents and aerosols Mobile Sources • Highway: cars, trucks, buses and motorcycles • Off-highway: aircraft, boats, farm equipment, and construction machinery.

Natural Sources • • • Forest fires- ash, particulates, carbon dioxide Volcanoes- ash, acid mist, hydrogen sulfide Decaying vegetation- sulfur cmpds Trees & bushes- Volatile Organic Cmpds (VOC’s) Pollen Viruses Bacteria Dust- from storms in arid regions Gut bacteria- methane gas

Anthropogenic Sources of Air Pollution

Criteria Air Pollutants EPA uses seven "criteria pollutants" as indicators of air quality 1. Sulfur Dioxide: SO 2 2. Nitrogen Dioxide: NO 2 3. Carbon monoxide: CO 4. Lead: Pb 5. Particulate Matter: PM 10 (PM 2. 5) 6. Volatile Organic Compounds: (VOCs) 7. Ozone: ground level O 3

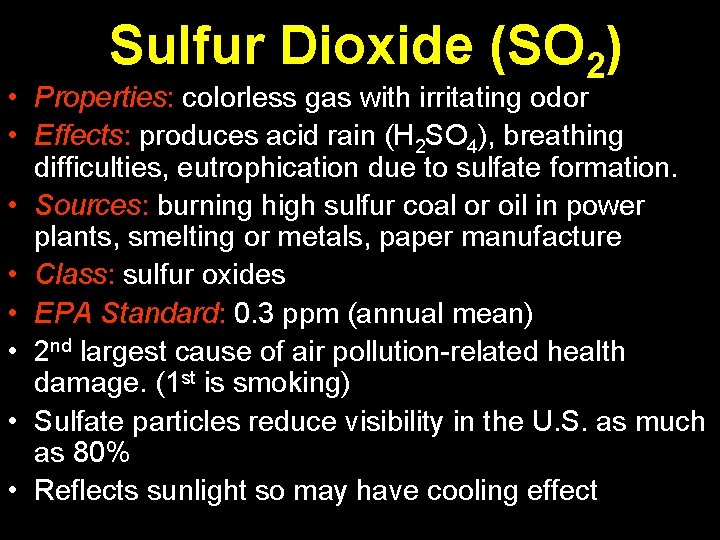
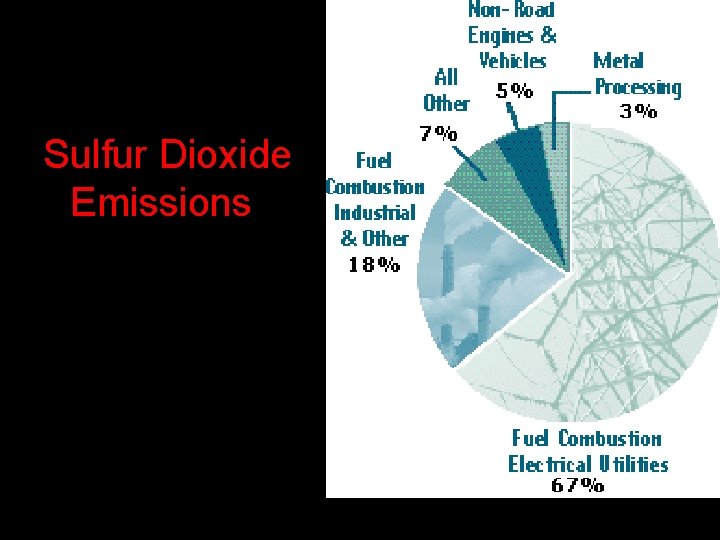
Sulfur Dioxide (SO 2) • Properties: colorless gas with irritating odor • Effects: produces acid rain (H 2 SO 4), breathing difficulties, eutrophication due to sulfate formation. • Sources: burning high sulfur coal or oil in power plants, smelting or metals, paper manufacture • Class: sulfur oxides • EPA Standard: 0. 3 ppm (annual mean) • 2 nd largest cause of air pollution-related health damage. (1 st is smoking) • Sulfate particles reduce visibility in the U. S. as much as 80% • Reflects sunlight so may have cooling effect

Sulfur Dioxide Emissions

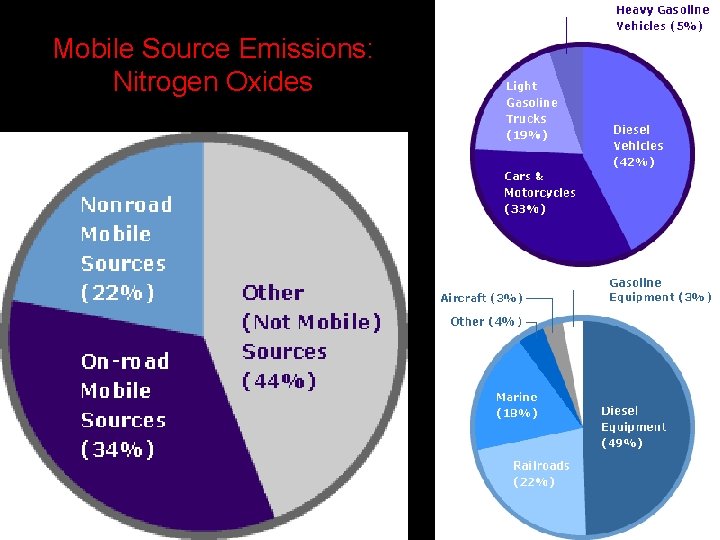
Nitrogen Dioxide (NO 2) • Properties: reddish brown gas, formed as fuel burned in car, strong oxidizing agent, forms Nitric acid (HNO 3) in air • Effects: acid rain, lung and heart problems, decreased visibility (yellow haze), suppresses plant growth • Sources: fossil fuels combustion, power plants, forest fires, volcanoes, bacteria in soil, fertilizers • Class: Nitrogen oxides (NOx) • EPA Standard: 0. 053 ppm • Excess nitrogen is causing fertilization & eutrophication of inland waters & seas

Mobile Source Emissions: Nitrogen Oxides

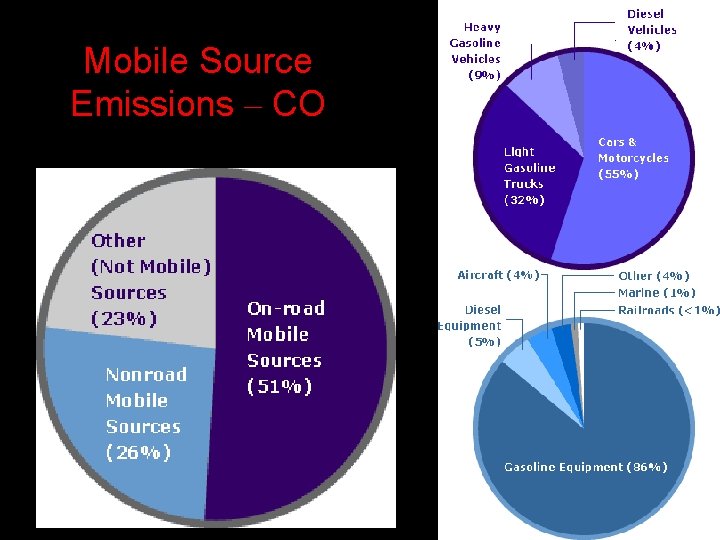
Carbon Monoxide (CO) • Properties: colorless, odorless, heavier than air, 0. 0036% of atmosphere • Effects: binds tighter to Hemoglobin (Hb) than O 2, so organs do not get O 2 needed, makes you sleepy, impairs mental functions and visual acuity, even at low levels • Sources: incomplete combustion of fossil fuels 60 95% from auto exhaust • Class: carbon oxides (CO 2, CO) • EPA Standard: 9 ppm • 1 billion tons enter atmosphere/year

Mobile Source Emissions – CO

Lead (Pb) • Properties: grayish metal • Effects: accumulates in tissue; affects kidneys, liver and nervous system (children most susceptible); mental retardation; possible carcinogen; 20% of inner city kids have high levels • Sources: particulates from fuel combustion, smelters, batteries • Class: toxic or heavy metals • EPA Standard: 1. 5 ug/m 3 • 2 million tons enter atmosphere/year • Mercury- neurotoxin from coal power plants • Both mercury & lead travel on air currents and fall into aquatic ecosystems causing bioaccumulation & biomagnification in food webs.

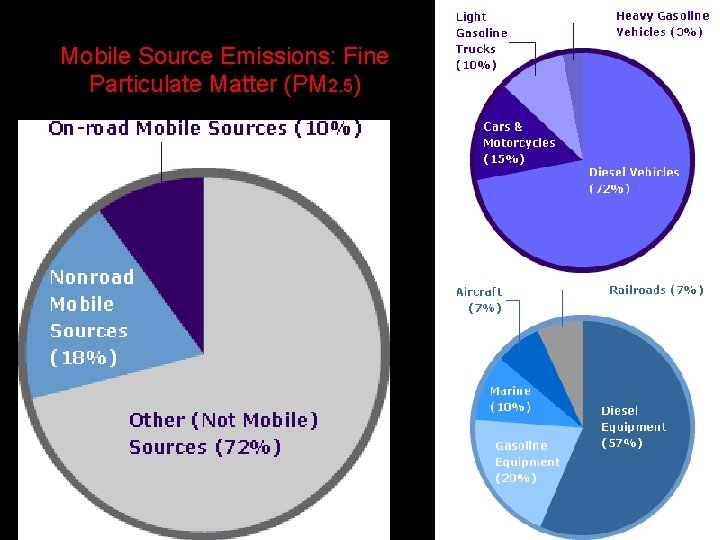
Suspended Particulate Matter (PM 10) • Properties: particles suspended in air (<10 um) • Effects: lung damage, mutagenic, carcinogenic, teratogenic • Sources: burning coal or diesel, volcanoes, factories, unpaved roads, plowing, lint, pollen, spores, burning fields • Class: SPM: dust, soot, asbestos, lead, PCBs (polychlorinated biphenyls), dioxins, pesticides • EPA Standard: 50 ug/m 3 (annual mean) • PM 2. 5 is worse because small enough to be inhaled more deeply • Asbestos fibers & cigarette smoke are most dangerous respirable particles because they are carcinogenic

Mobile Source Emissions: Fine Particulate Matter (PM 2. 5)

VOCs (Volatile Organic Compounds) • Properties: organic compounds (hydrocarbons) that evaporate easily, usually aromatic • Effects: eye and respiratory irritants; carcinogenic; liver, CNS, or kidney damage; damages plants; lowered visibility due to brown haze; global warming • Sources: vehicles (largest source), evaporation of solvents or fossil fuels, aerosols, paint thinners, dry cleaning, wetlands, rice paddies, bacteria, plants. • Class: HAPs (Hazardous Air Pollutants- cause cancer, birth defects, mutation, neutroxins) – Methane – Benzene – Chlorofluorocarbons (CFCs), etc. • Concentrations indoors up to 1000 x outdoors • 600 million tons of CFCs

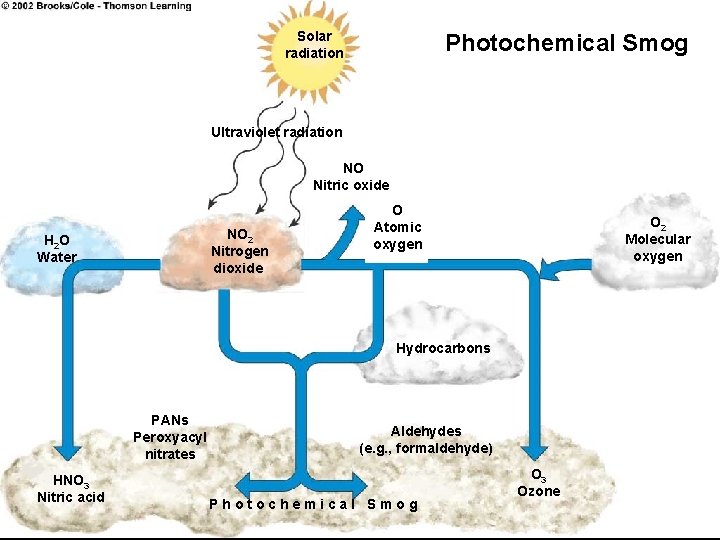
Ozone (O 3) • Properties: colorless, unpleasant odor, major part of photochemical smog • Effects: lung irritant, damages plants, rubber, fabric, eyes • Sources: Created by sunlight acting on NOx and VOC , photocopiers, cars, industry, gas vapors, chemical solvents, incomplete fuel combustion products • Class: photochemical oxidants • Good ozone vs. bad ozone- good is in stratosphere and bad is at ground level (from cars) • Figure 18. 10 shows secondary production of urban smog by photochemical rxns in atmosphere


Other Air Pollutants • Carbon dioxide- natural source from photosynthesis & respiration; human caused from fossil fuels & deforestation • Chloro. Fluoro. Carbons (CFC’s)- from refrigerants, aerosols, Styrofoam • Formaldehyde- building materials & household products • Benzene- paint • Asbestos- car brakes, building materials • Dioxins- pesticides • Cadmium- batteries, plastics industry

Formation & Intensity of Pollutant is influenced by… • Local climate (inversions, air pressure, temperature, humidity) • Topography (hills and mountains) • Population density • Amount of industry • Fuels used by population and industry for heating, manufacturing, transportation, power • Weather: rain, snow, wind • Buildings (slow wind speed) • Mass transit used

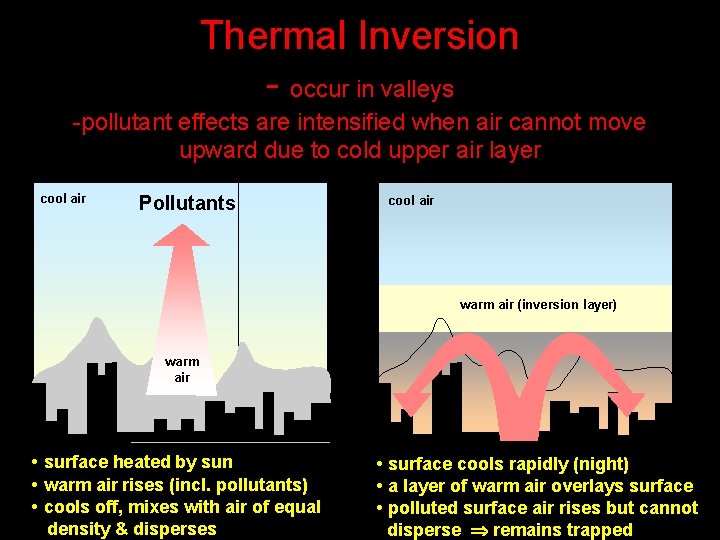
Thermal Inversion - occur in valleys -pollutant effects are intensified when air cannot move upward due to cold upper air layer cool air Pollutants cool air warm air (inversion layer) warm air • surface heated by sun • warm air rises (incl. pollutants) • cools off, mixes with air of equal density & disperses • surface cools rapidly (night) • a layer of warm air overlays surface • polluted surface air rises but cannot disperse remains trapped

Smog Forms. . . when polluted air is stagnant (weather conditions, geographic location) Los Angeles, CA

Solar radiation Photochemical Smog Ultraviolet radiation NO Nitric oxide NO 2 Nitrogen dioxide H 2 O Water O Atomic oxygen O 2 Molecular oxygen Hydrocarbons PANs Peroxyacyl nitrates HNO 3 Nitric acid Aldehydes (e. g. , formaldehyde) Photochemical Smog O 3 Ozone

Urban Heat Islands • Cities are generally 3 -5ºC warmer than rural areas • Caused by: – Lack of vegetation to absorb heat – Dark buildings & roads trap heat – Buildings create windbreaks • Dust Dome- trapping of dirt & particulates over city

INDOOR AIR POLLUTION

What are some sources of indoor air pollution? 1. Cigarette smoke – – Deadliest indoor air pollutant Contain formaldehyde, carbon monoxide Causes lung cancer, emphysema Second hand smoke may be worse due to particulates that come from tip.

What are some sources of indoor air pollution? 2. Mold – Moisture in carpets – Allergy symptoms, breathing problems, headache, fatigue

What are some sources of indoor air pollution? 3. Carbon monoxide – Malfunctioning furnace, gas appliances, cars – Blood cannot carry oxygen – Feel sleepy, nausea, dizzy, cause death.

What are some sources of indoor air pollution? 4. Radon – – – Colorless, odorless, radioactive gas Comes from soil under basements Long term exposure can cause lung cancer Fix cracks in floor or walls to prevent influx of radon Install ventilation fan in basement to blow radon out. Zone 1 (purple) high levels of radon Zone 3 (yellow) low levels of radon

What are some sources of indoor air pollution? 5. Asbestos – Roofing, flooring, insulation, brakes – OK… unless disturbed or deteriorates – Can cause asbestosis (scarring of lungs) and mesothelioma (type of lung cancer) Plaque build up (scarring) in lung w/asbestosis

What are some sources of indoor air pollution? 6. Lead – Old homes, toys, lead crystal dishes – Causes behavior & learning problems, slow growth, hearing problems, headaches

What are some sources of indoor air pollution? 7. Formaldehyde – Pressed wood, paneling, particle board, glue. – Respiratory irritation, fatigue, skin rash, known to cause cancer

What are some sources of indoor air pollution? 8. VOC’s – Paradichlorobenzenemothballs, insecticides – (perchloroethylene))- dry cleaned clothes – Benzene- paints, cigarettes – Causes respiratory problems, headaches, loss of coordination, nausea, organ damage, cancer

Effects of Air Pollution on… 1. Human Health 2. Plant Health 3. Acid Deposition

1. Human Health • Depends on intensity & duration of exposure, age & prior health status • At-risk groups: young, old, or already suffering from respiratory/cardiovascular disease. Also, more active & outside vs. sedentary inside lifestyle • Most susceptible- less-developed countries use smoky fires for cooking & heating

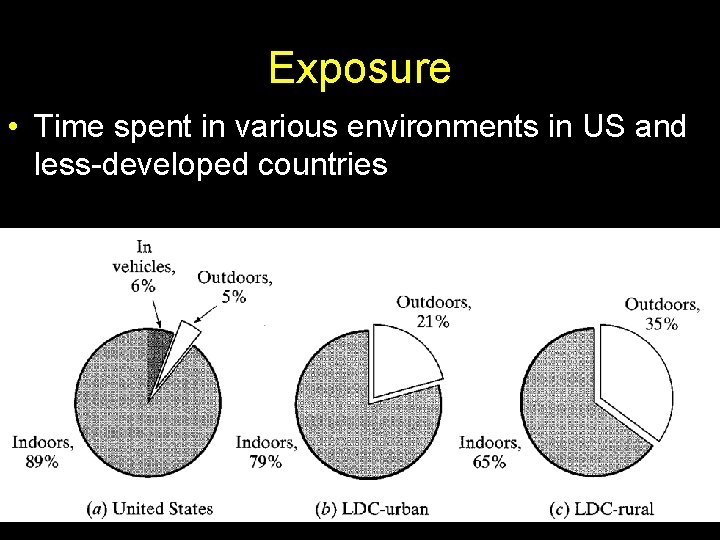
Exposure • Time spent in various environments in US and less-developed countries

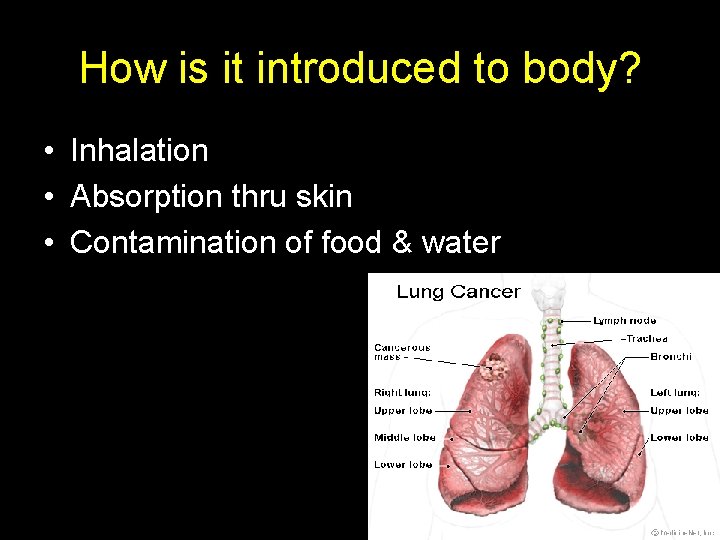
How is it introduced to body? • Inhalation • Absorption thru skin • Contamination of food & water

How does air pollution affect people? • Chronic bronchitiscoughing, trouble breathing • Asthma- not caused by air pollution, but aggravated by it. • Emphysema- lungs lose elasticity, hard to breathe • Lung Cancer- caused by cigarettes, car exhaust, particulates, asbestos, arsenic, radon

§ How does air pollution Sick building syndrome- affect people? – Buildings closed up to save energy- no circulation – Effects of fumes intensified – Symptoms: headache, eye or throat irritation, cough, itchy skin, dizziness, nausea, fatigue – Feel better when you get fresh air outside. – ≥ 20% of workers must be afflicted to be classified as SBS

2. Plant Health • Two Methods of Damage – Directly toxic • Irritate cell membranes • First few days- discoloration due to chlorosis (bleaching) of leaf • Later- necrotic (dead) lesions develop leading to death – Disruption of plant hormones • Ethylene from fossil fuels, chemical plants is a major culprit • Synergistic effects (when combined two are worse than each individually) unpredictable – White pine seedlings exposed to low levels of O 3 & SO 2 individually are fine. When combined cause death – In alfalfa, O 3 and SO 2 together are less harmful than individually. • Air pollutant effects on plants are sometimes confused with insect damage or other diseases.

Necrosis of watermelon leaf Necrotic lesions on lower surface of potato leaves

3. Acid Deposition

Measuring Acid Rain • Normal rain is slightly acidic and has a p. H of about 5. 0 -5. 6 • Any rainfall with a p. H value less than 5. 0 is defined as acid rain

Two Forms… • Wet Refers to acid rain, fog, sleet, cloud vapor and snow. • Dry Refers to acidic gases and particles.

Increased Acidity • Dry deposited gases and particles can also be washed from trees and other surfaces by rainstorms. • The runoff water adds those acids to the acid rain, making the combination more acidic than the falling rain alone.

Compounds Two main contributers to acid deposition: • Sulfur Dioxide (SO 2) • Nitrogen Oxides (NOx) – NO- nitric oxide (or nitrogen monoxide) – NO 2 - nitrogen dioxide – N 2 O- nitrous oxide • 66% of all sulfur dioxides and 25% of all nitrogen oxides comes from coal or oil electric power plants. Most nitrogen oxides come from cars

When gas pollutants e. g. sulfur dioxide, nitrogen dioxide dissolve in rain water, various acids are formed. CO 2 + H 2 O SO 2 + H 2 O NO 2 + H 2 O H 2 CO 3 (carbonic acid) H 2 SO 4 (sulfuric acid) HNO 2 (nitrous acid) + HNO 3 (nitric acid)

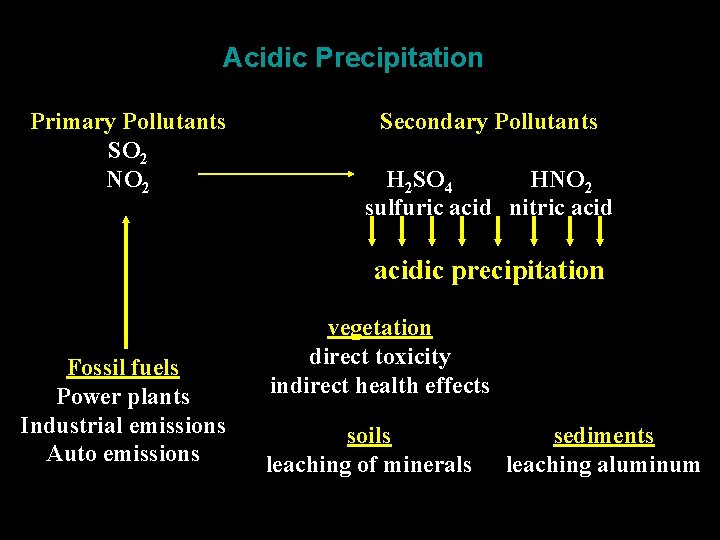
Acidic Precipitation Primary Pollutants SO 2 NO 2 Secondary Pollutants H 2 SO 4 HNO 2 sulfuric acid nitric acidic precipitation Fossil fuels Power plants Industrial emissions Auto emissions vegetation direct toxicity indirect health effects soils leaching of minerals sediments leaching aluminum


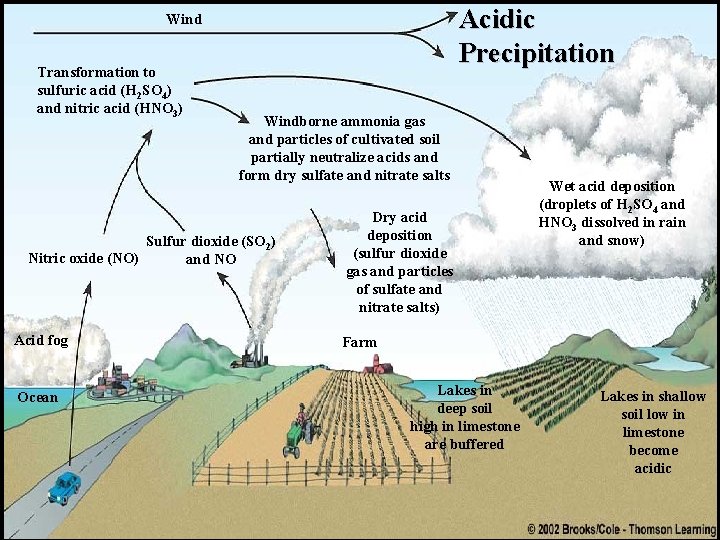
Acidic Precipitation Wind Transformation to sulfuric acid (H 2 SO 4) and nitric acid (HNO 3) Windborne ammonia gas and particles of cultivated soil partially neutralize acids and form dry sulfate and nitrate salts Sulfur dioxide (SO 2) Nitric oxide (NO) and NO Acid fog Ocean Dry acid deposition (sulfur dioxide gas and particles of sulfate and nitrate salts) Wet acid deposition (droplets of H 2 SO 4 and HNO 3 dissolved in rain and snow) Farm Lakes in deep soil high in limestone are buffered Lakes in shallow soil low in limestone become acidic

Effects of Acid Rain • The strength of the effects depend on many factors – How acidic the water is – The types of fish, trees, and other living things that rely on the water – The chemistry and buffering capacity of the soils involved • limestone & basalt have high buffering capacity • have high ANC (Acid Neutralizing Capacity)

Effects of Acid Rain • Has a variety of effects, including damage to forests and soils, fish and other living things, materials, and human health. • Also reduces how far and how clearly we can see through the air, an effect called visibility reduction. • Effects of acid rain are most clearly seen in the aquatic environments • Most lakes and streams have a p. H between 6 and 8 http: //cica. indiana. edu/projects/Biology/movies. html

Buffering Capacity • Acid rain primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited “buffering capacity” (places that have granite bedrock or soil for example) • Lakes and streams become acidic when the water itself and its surrounding soil cannot buffer the acid rain enough to neutralize it.

• In areas where buffering capacity is low, acid rain also releases aluminum from soils into lakes and streams • aluminum is highly toxic to many species of aquatic organisms. – Can attach to fish gills causing suffocation – Can release from soil particles & enter solutions taken up by plants causing death http: //home. earthlink. net/~photofish/fish_photos/sw 10_thumb. jpg

Effects on Wildlife • Some birds have left areas- no fish, forests destroyed- less nesting space • Young of most species are more sensitive to environmental conditions than adults. • At p. H 5, most fish eggs cannot hatch. • At lower p. H levels, some adult fish die. • Both low p. H and increased aluminum levels are directly toxic to fish. – Can also stress fish resulting in low body weight, small size, less able to compete for food, habitats, reduced reproduction, increased susceptibility to disease Loons no longer nesting in Adirondack Mtn lakes- too acidic for fish which they eat Salmon populations have decreased in Norway since 1950 due to acid rain. Red areas show where populations have declined.

Acid Rain and Forests • Acid rain does not usually kill trees directly. • Instead, it is more likely to – weaken trees by damaging their leaves – limit the nutrients available to them – expose them to toxic substances slowly released from the soil.

Acid Rain & Forests • Trees at higher elevations can be more effected because of increased exposure to acid fog or acid cloud vapor • As water evaporates from leaf, acid becomes more concentrated, burning the leaf tissue.

Effects of Acid Rain Great Smoky Mountains, NC

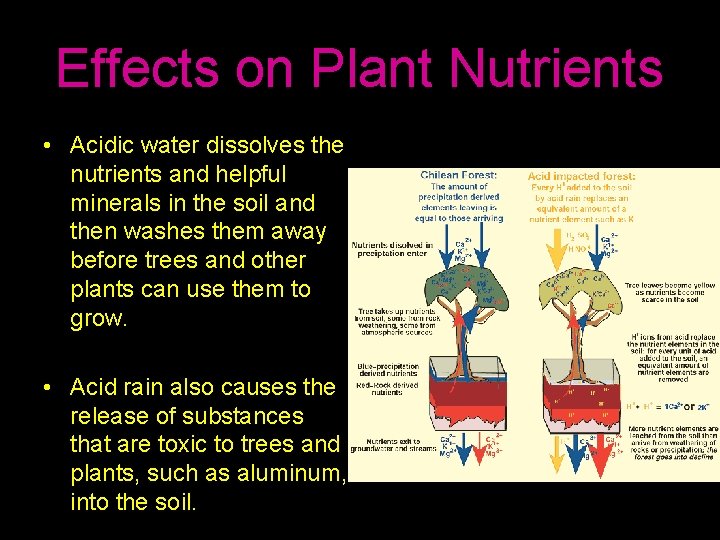
Effects on Plant Nutrients • Acidic water dissolves the nutrients and helpful minerals in the soil and then washes them away before trees and other plants can use them to grow. • Acid rain also causes the release of substances that are toxic to trees and plants, such as aluminum, into the soil.

Effects on Property • Many statues, monuments, etc. made from limestone (Ca. CO 3), marble or metal • Acid rain can dissolve rock or tarnish metal • Expensive to restore, refurbish, maintain • Car manufacturers now use acid-resistant paint at a cost of $5. 00 per new vehicle
 Control of particulate matter
Control of particulate matter Advantages and disadvantages of train
Advantages and disadvantages of train Fiberpharm
Fiberpharm Chapter 12 air section 1
Chapter 12 air section 1 Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Conclusion of sound pollution
Conclusion of sound pollution Radon indoor air pollution
Radon indoor air pollution Air pollution 2050
Air pollution 2050 Northern sonoma county air pollution control district
Northern sonoma county air pollution control district Effect of air pollution on plants
Effect of air pollution on plants Pollution control laws in india
Pollution control laws in india Indoor air pollution sources
Indoor air pollution sources Ari rokeach
Ari rokeach Air pollution conclusion
Air pollution conclusion Air pollution wildfires
Air pollution wildfires Primary and secondary pollutants
Primary and secondary pollutants Air pollution
Air pollution Two sources of air pollution
Two sources of air pollution Definition of pollution in simple words
Definition of pollution in simple words Erg (air pollution control) ltd
Erg (air pollution control) ltd Example of environmental sustainability
Example of environmental sustainability Section 2 air noise and light pollution
Section 2 air noise and light pollution Man made resources examples
Man made resources examples Air pollution
Air pollution Contents of air pollution
Contents of air pollution Brainpop air pollution
Brainpop air pollution Air pollution
Air pollution Air pollution causing “dead area of leaf” is called
Air pollution causing “dead area of leaf” is called Air pollution box model example
Air pollution box model example Air pollution specialist
Air pollution specialist Air pollution
Air pollution Air pollution wildfires
Air pollution wildfires 5 effects of air pollution
5 effects of air pollution Land water and air pollution
Land water and air pollution Indoor air pollution examples
Indoor air pollution examples Air pollution
Air pollution Air pollution mexico
Air pollution mexico Mobile source definition
Mobile source definition Prevention of indoor air pollution
Prevention of indoor air pollution Air pollution aims and objectives
Air pollution aims and objectives Air pollution control methods
Air pollution control methods What is inorganic pollution
What is inorganic pollution General effects of air pollution
General effects of air pollution Aim and objectives of air pollution
Aim and objectives of air pollution Thematic strategy on air pollution
Thematic strategy on air pollution Air pollution
Air pollution Air pollution box model example
Air pollution box model example Introduction about air pollution
Introduction about air pollution Air pollution simulator
Air pollution simulator Objectives of air pollution
Objectives of air pollution Section 2 air noise and light pollution
Section 2 air noise and light pollution Adjectives for air pollution
Adjectives for air pollution Air pollution
Air pollution Conclusion of air pollution
Conclusion of air pollution Main cause of air pollution
Main cause of air pollution Air pollution
Air pollution Objectives for pollution
Objectives for pollution Effects of land pollution on human health
Effects of land pollution on human health Air pollution consequences
Air pollution consequences Air pollution control technology
Air pollution control technology Denah sanitasi air bersih dan air kotor
Denah sanitasi air bersih dan air kotor Right hand in the air left hand in the air
Right hand in the air left hand in the air Single acting cylinder example
Single acting cylinder example