Advanced Electronics Materials Cyclotene Advanced Electronics Resins CH




- Slides: 37

Advanced Electronics Materials Cyclotene* Advanced Electronics Resins CH 3 Si CH 3 O Si CH 3 Information on Adhesion Science applied to Organic – Inorganic Thin Films Jay Im, Ted Stokich, Phil Garrou, Ed Harris, Gary Buske, Ed Shaffer, Paul Townsend, Brian Martin, Mike Radler, Tom Wells, Bob Heistand, Andy Strandjord, Jim Curphy, Jack Hetzner, Dan Scheck, Albert Achen AA Sept 05 *Trademark of The Dow Chemical Company 1

Advanced Electronics Materials Index 1. BCB Adhesion on Inorganics 1. Basics 2. Adhesion Promoters 3. AP 3000 Performance 4. Adhesion Testing 2. Metal on BCB Adhesion 3. Potential Issues of Si based Interfaces AA Sept 05 *Trademark of The Dow Chemical Company 2

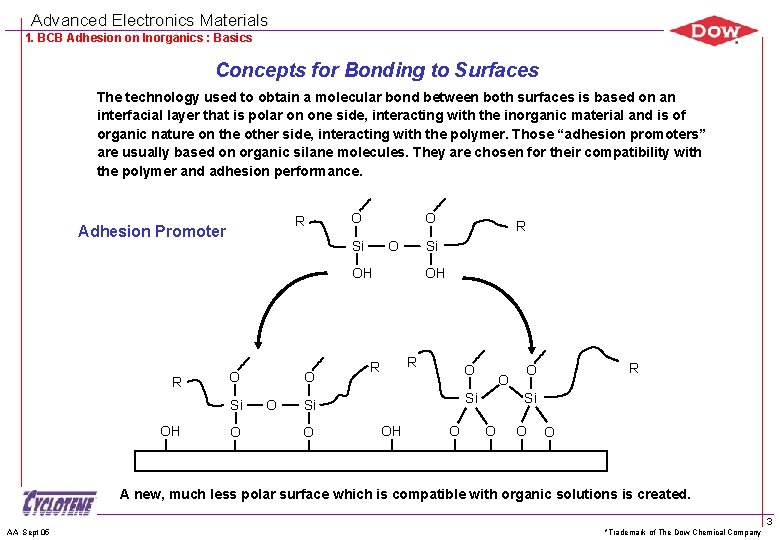
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Basics Concepts for Bonding to Surfaces The technology used to obtain a molecular bond between both surfaces is based on an interfacial layer that is polar on one side, interacting with the inorganic material and is of organic nature on the other side, interacting with the polymer. Those “adhesion promoters” are usually based on organic silane molecules. They are chosen for their compatibility with the polymer and adhesion performance. R Adhesion Promoter O O Si OH R O Si OH O O O R OH R R O Si Si O O OH O R O Si O O O A new, much less polar surface which is compatible with organic solutions is created. AA Sept 05 *Trademark of The Dow Chemical Company 3

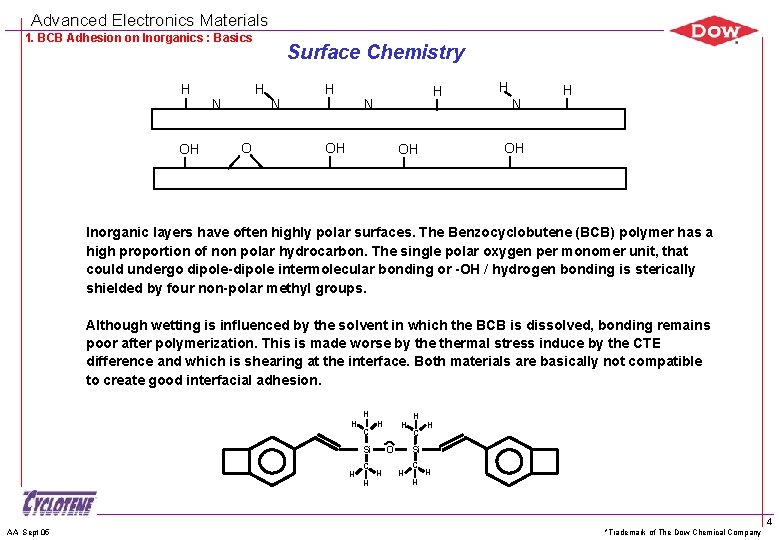
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Basics H OH Surface Chemistry H N O N H H N OH H N H OH OH Inorganic layers have often highly polar surfaces. The Benzocyclobutene (BCB) polymer has a high proportion of non polar hydrocarbon. The single polar oxygen per monomer unit, that could undergo dipole-dipole intermolecular bonding or -OH / hydrogen bonding is sterically shielded by four non-polar methyl groups. Although wetting is influenced by the solvent in which the BCB is dissolved, bonding remains poor after polymerization. This is made worse by thermal stress induce by the CTE difference and which is shearing at the interface. Both materials are basically not compatible to create good interfacial adhesion. Si O H H H AA Sept 05 C H H C H Si H C H H *Trademark of The Dow Chemical Company 4

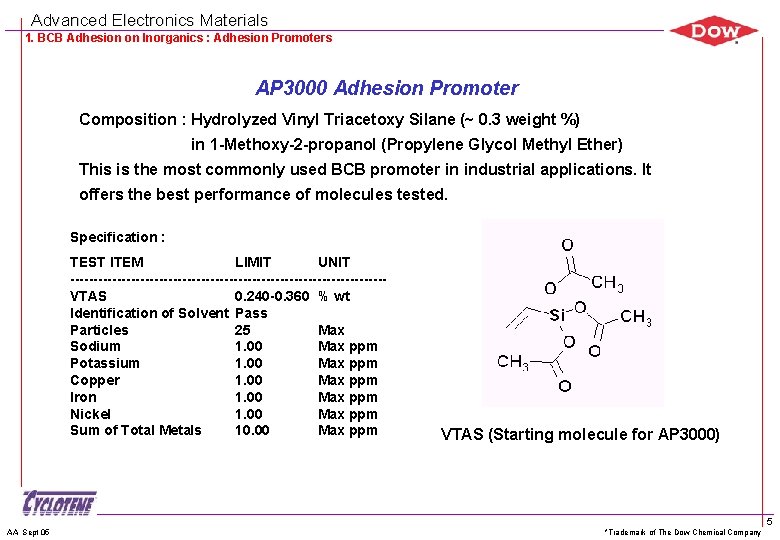
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Promoters AP 3000 Adhesion Promoter Composition : Hydrolyzed Vinyl Triacetoxy Silane (~ 0. 3 weight %) in 1 -Methoxy-2 -propanol (Propylene Glycol Methyl Ether) This is the most commonly used BCB promoter in industrial applications. It offers the best performance of molecules tested. Specification : TEST ITEM LIMIT UNIT ----------------------------------VTAS 0. 240 -0. 360 % wt Identification of Solvent Pass Particles 25 Max Sodium 1. 00 Max ppm Potassium 1. 00 Max ppm Copper 1. 00 Max ppm Iron 1. 00 Max ppm Nickel 1. 00 Max ppm Sum of Total Metals 10. 00 Max ppm AA Sept 05 VTAS (Starting molecule for AP 3000) *Trademark of The Dow Chemical Company 5

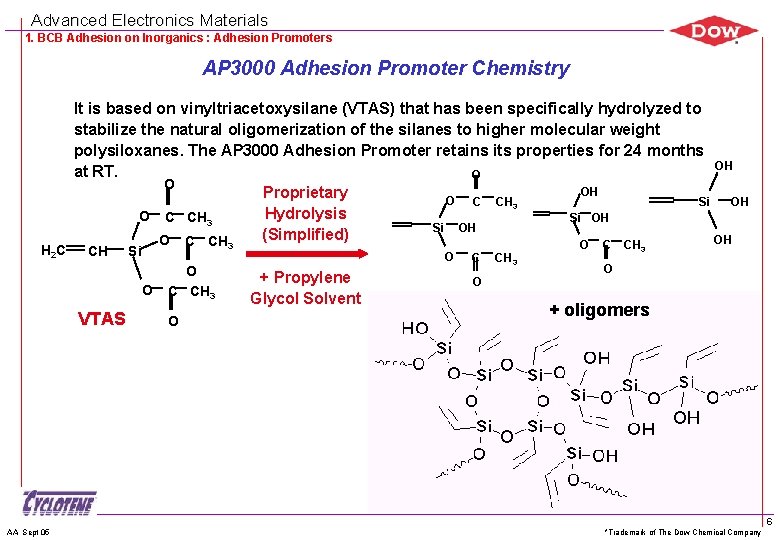
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Promoters AP 3000 Adhesion Promoter Chemistry H 2 C It is based on vinyltriacetoxysilane (VTAS) that has been specifically hydrolyzed to stabilize the natural oligomerization of the silanes to higher molecular weight polysiloxanes. The AP 3000 Adhesion Promoter retains its properties for 24 months OH at RT. O O OH Proprietary O C CH 3 Si OH Hydrolysis O C CH 3 Si OH (Simplified) OH O C CH CH 3 Si O VTAS AA Sept 05 O C CH 3 O O + Propylene Glycol Solvent C CH 3 O O + oligomers *Trademark of The Dow Chemical Company 6

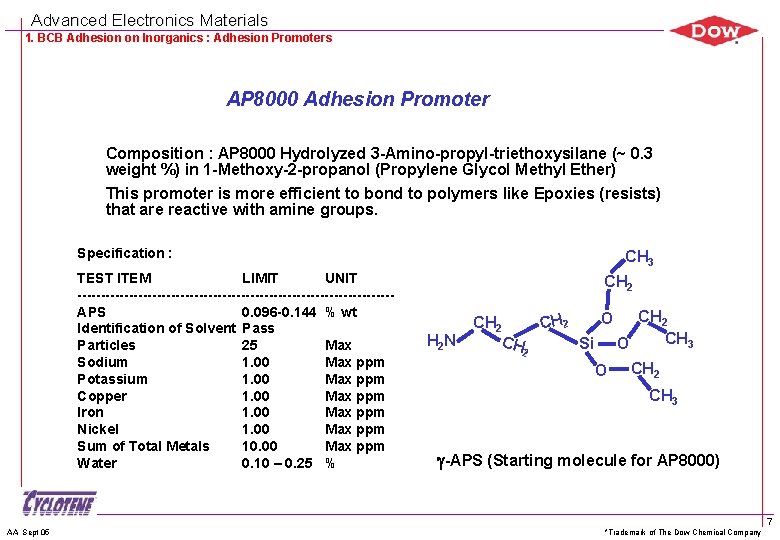
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Promoters AP 8000 Adhesion Promoter Composition : AP 8000 Hydrolyzed 3 -Amino-propyl-triethoxysilane (~ 0. 3 weight %) in 1 -Methoxy-2 -propanol (Propylene Glycol Methyl Ether) This promoter is more efficient to bond to polymers like Epoxies (resists) that are reactive with amine groups. Specification : TEST ITEM LIMIT UNIT ----------------------------------APS 0. 096 -0. 144 % wt Identification of Solvent Pass Particles 25 Max Sodium 1. 00 Max ppm Potassium 1. 00 Max ppm Copper 1. 00 Max ppm Iron 1. 00 Max ppm Nickel 1. 00 Max ppm Sum of Total Metals 10. 00 Max ppm Water 0. 10 – 0. 25 % AA Sept 05 CH 3 CH 2 N CH 2 O CH 2 Si O CH 2 CH 3 g-APS (Starting molecule for AP 8000) *Trademark of The Dow Chemical Company 7

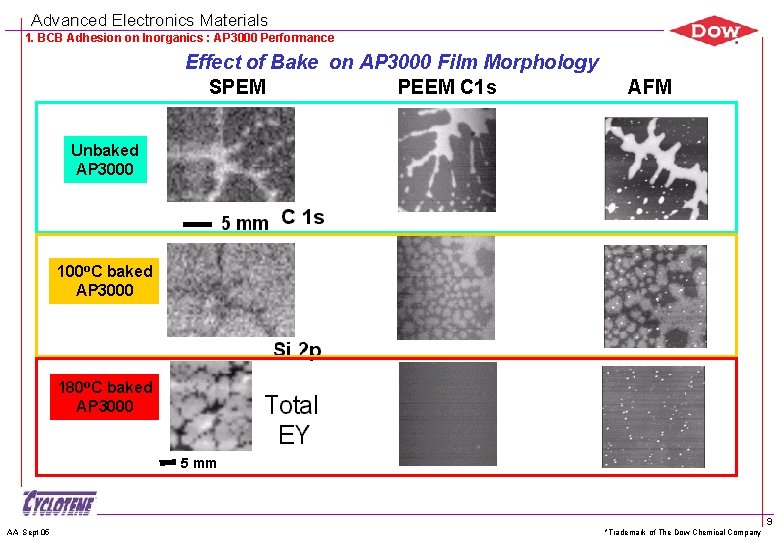
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : AP 3000 Performance Structure of Adhesion Promoters Films from X-Ray Photoemission Electron Microscopy Terminology n n n SPEM = Scanning photoelectron microscopy PEEM = photoemission electron microscopy SPEM and PEEM are new tools useful for determining surface chemistry on submicron lateral spatial scale Summary of findings n n AP 3000 films formed ridges observed by AFM, SPEM and PEEM Ridges were not due to dewetting but substantial AP between them Reference: G. E. Mitchell 1 a, A. P. Hitchcock 2, T. M. Stokich Jr. 1 b, A. Scholl 3, S. Anders 3, R. Graupner 3, G. F. Meyers 1 a, E. G. Rightor 1 a, D. D. Hawn 1 a, J-H. Im 1 b , E. O. Shaffer II 1 b, T. Warwick 3, Characterization of Adhesion Promoter Films Using PEEM and SPEM, to be published in the Advanced Light Source Compendium of User Abstracts for 1999 AA Sept 05 *Trademark of The Dow Chemical Company 8

Advanced Electronics Materials 1. BCB Adhesion on Inorganics : AP 3000 Performance Effect of Bake on AP 3000 Film Morphology SPEM PEEM C 1 s AFM Unbaked AP 3000 100 o. C baked AP 3000 180 o. C baked AP 3000 5 mm AA Sept 05 *Trademark of The Dow Chemical Company 9

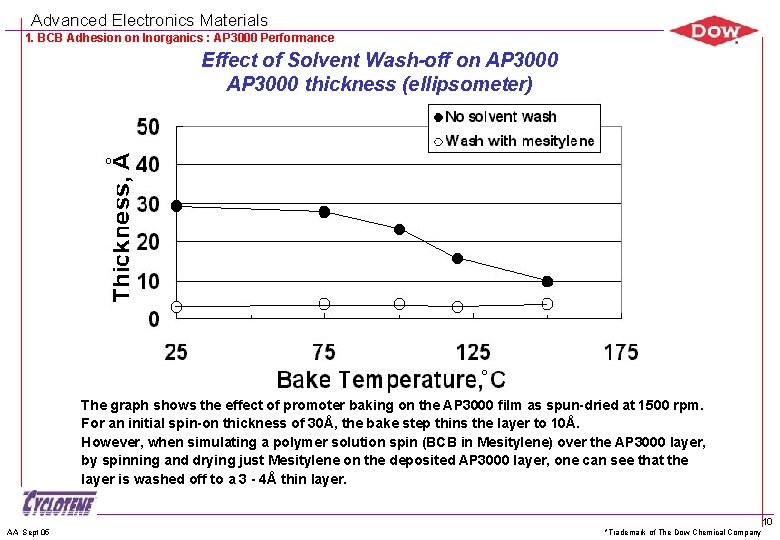
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : AP 3000 Performance Effect of Solvent Wash-off on AP 3000 thickness (ellipsometer) The graph shows the effect of promoter baking on the AP 3000 film as spun-dried at 1500 rpm. For an initial spin-on thickness of 30Å, the bake step thins the layer to 10Å. However, when simulating a polymer solution spin (BCB in Mesitylene) over the AP 3000 layer, by spinning and drying just Mesitylene on the deposited AP 3000 layer, one can see that the layer is washed off to a 3 - 4Å thin layer. AA Sept 05 *Trademark of The Dow Chemical Company 10

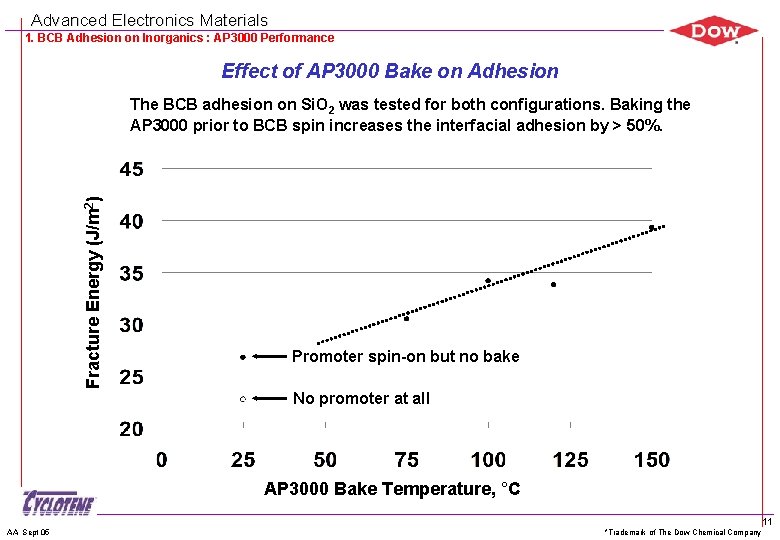
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : AP 3000 Performance Effect of AP 3000 Bake on Adhesion Fracture Energy (J/m 2) The BCB adhesion on Si. O 2 was tested for both configurations. Baking the AP 3000 prior to BCB spin increases the interfacial adhesion by > 50%. Promoter spin-on but no bake No promoter at all AP 3000 Bake Temperature, °C AA Sept 05 *Trademark of The Dow Chemical Company 11

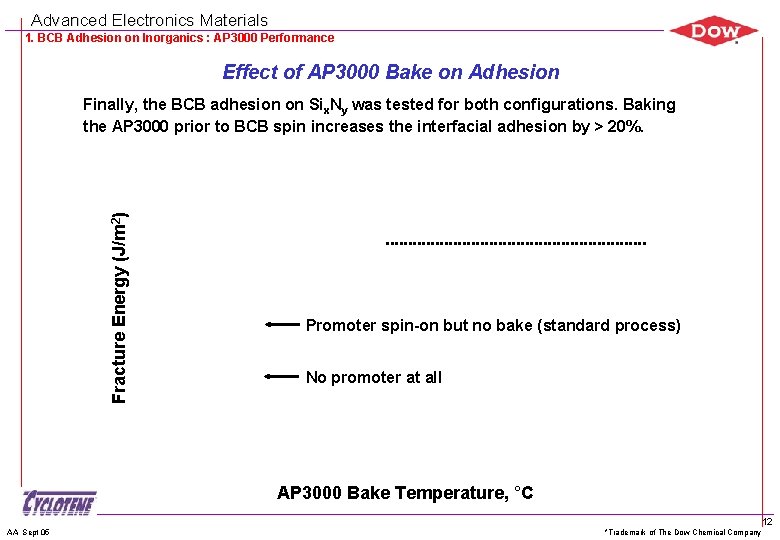
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : AP 3000 Performance Effect of AP 3000 Bake on Adhesion Fracture Energy (J/m 2) Finally, the BCB adhesion on Six. Ny was tested for both configurations. Baking the AP 3000 prior to BCB spin increases the interfacial adhesion by > 20%. Promoter spin-on but no bake (standard process) No promoter at all AP 3000 Bake Temperature, °C AA Sept 05 *Trademark of The Dow Chemical Company 12

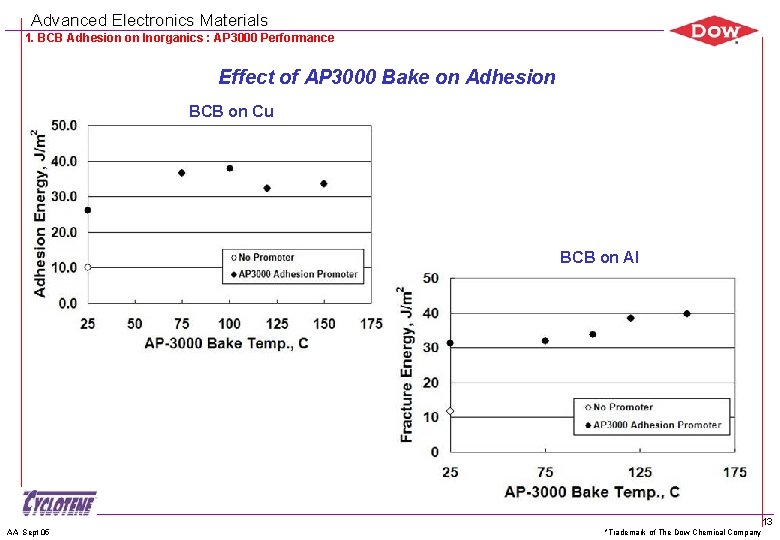
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : AP 3000 Performance Effect of AP 3000 Bake on Adhesion BCB on Cu BCB on Al AA Sept 05 *Trademark of The Dow Chemical Company 13

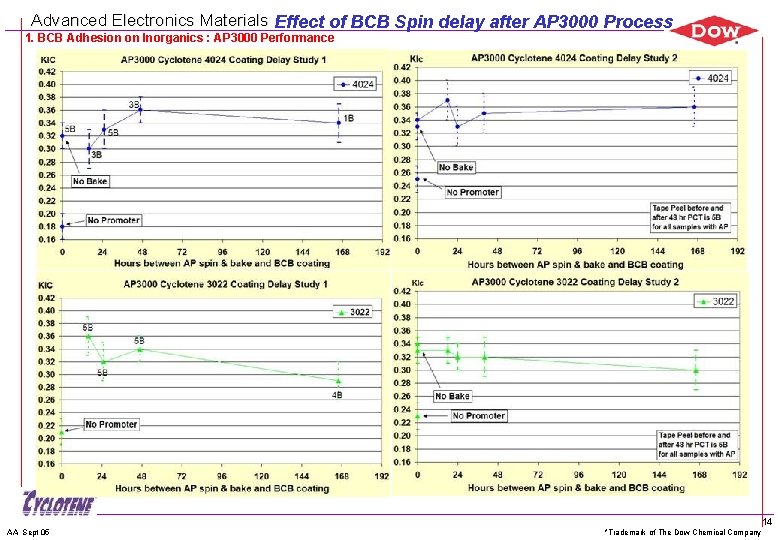
Advanced Electronics Materials Effect of BCB Spin delay after AP 3000 Process 1. BCB Adhesion on Inorganics : AP 3000 Performance AA Sept 05 *Trademark of The Dow Chemical Company 14

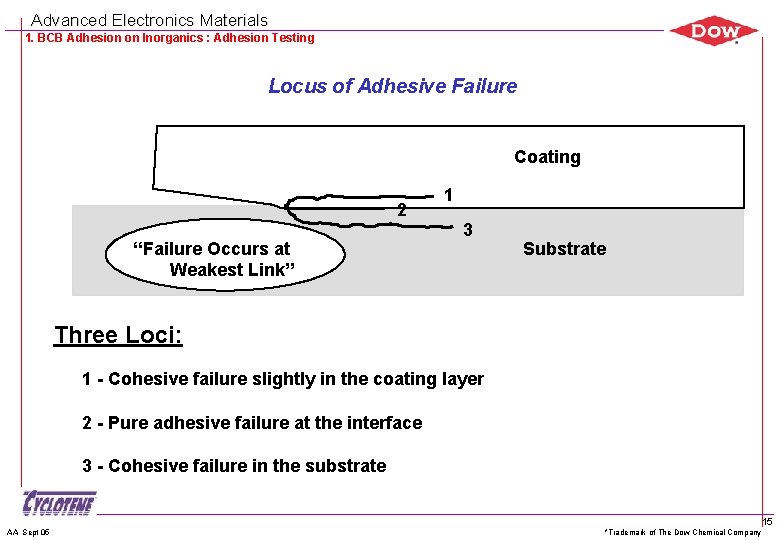
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Locus of Adhesive Failure Coating 2 “Failure Occurs at Weakest Link” 1 3 Substrate Three Loci: 1 - Cohesive failure slightly in the coating layer 2 - Pure adhesive failure at the interface 3 - Cohesive failure in the substrate AA Sept 05 *Trademark of The Dow Chemical Company 15

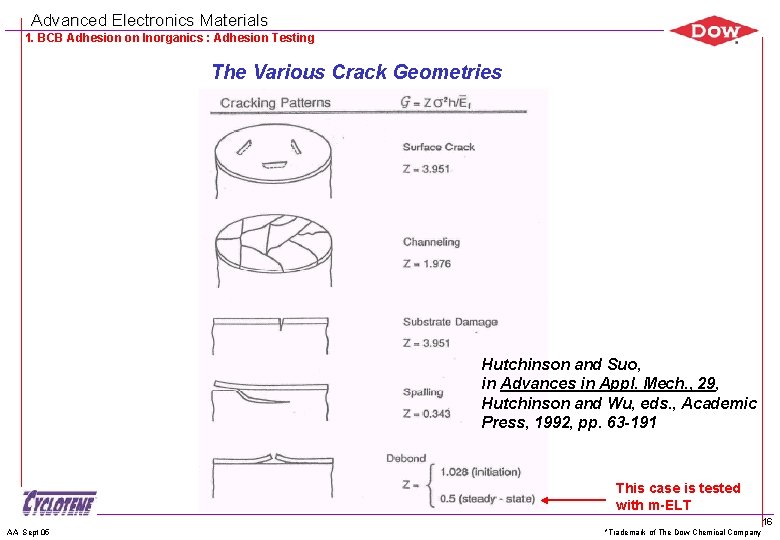
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing The Various Crack Geometries Hutchinson and Suo, in Advances in Appl. Mech. , 29, Hutchinson and Wu, eds. , Academic Press, 1992, pp. 63 -191 This case is tested with m-ELT AA Sept 05 *Trademark of The Dow Chemical Company 16

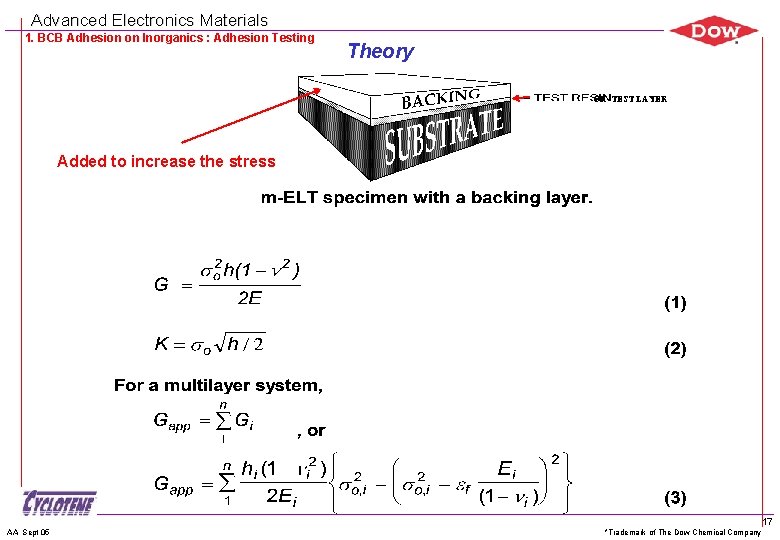
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Theory OR TEST LAYER Added to increase the stress AA Sept 05 *Trademark of The Dow Chemical Company 17

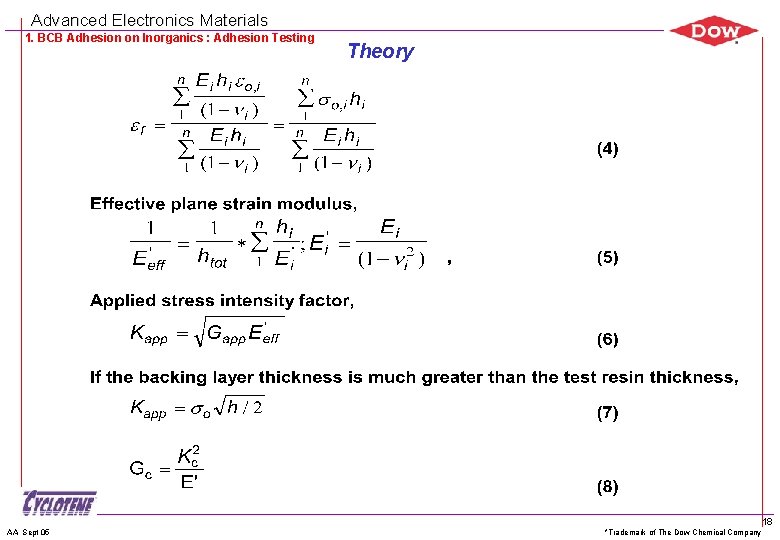
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing AA Sept 05 Theory *Trademark of The Dow Chemical Company 18

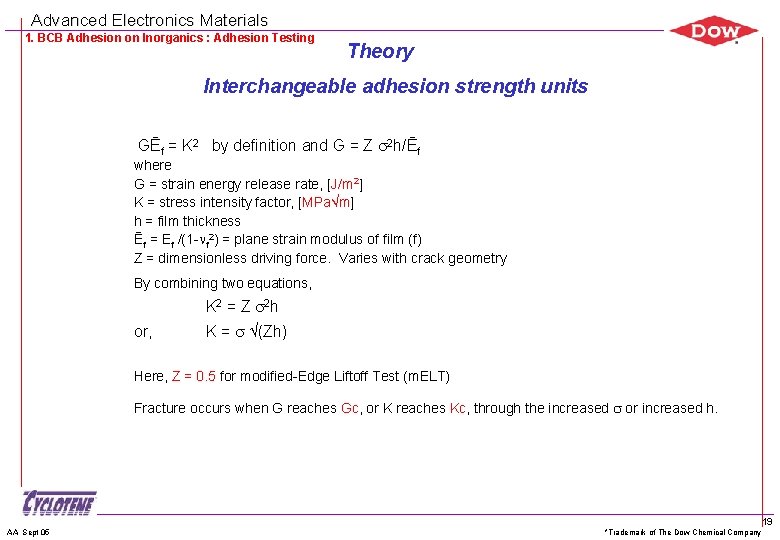
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Theory Interchangeable adhesion strength units GĒf = K 2 by definition and G = Z 2 h/Ēf where G = strain energy release rate, [J/m 2] K = stress intensity factor, [MPa m] h = film thickness Ēf = Ef /(1 - f 2) = plane strain modulus of film (f) Z = dimensionless driving force. Varies with crack geometry By combining two equations, K 2 = Z 2 h or, K = (Zh) Here, Z = 0. 5 for modified-Edge Liftoff Test (m. ELT) Fracture occurs when G reaches Gc, or K reaches Kc, through the increased or increased h. AA Sept 05 *Trademark of The Dow Chemical Company 19

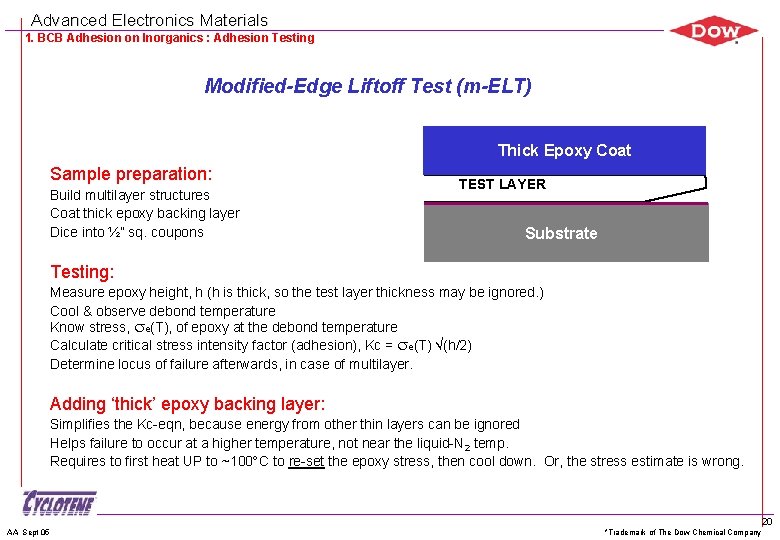
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Modified-Edge Liftoff Test (m-ELT) Thick Epoxy Coat Sample preparation: Build multilayer structures Coat thick epoxy backing layer Dice into ½” sq. coupons TEST LAYER Substrate Testing: Measure epoxy height, h (h is thick, so the test layer thickness may be ignored. ) Cool & observe debond temperature Know stress, e(T), of epoxy at the debond temperature Calculate critical stress intensity factor (adhesion), Kc = e(T) (h/2) Determine locus of failure afterwards, in case of multilayer. Adding ‘thick’ epoxy backing layer: Simplifies the Kc-eqn, because energy from other thin layers can be ignored Helps failure to occur at a higher temperature, not near the liquid-N 2 temp. Requires to first heat UP to ~100 C to re-set the epoxy stress, then cool down. Or, the stress estimate is wrong. AA Sept 05 *Trademark of The Dow Chemical Company 20

Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing m-ELT Tester AA Sept 05 *Trademark of The Dow Chemical Company 21

Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing m-ELT Tester (top view) Temperature Recorder N 2 cooled cryostage AA Sept 05 *Trademark of The Dow Chemical Company 22

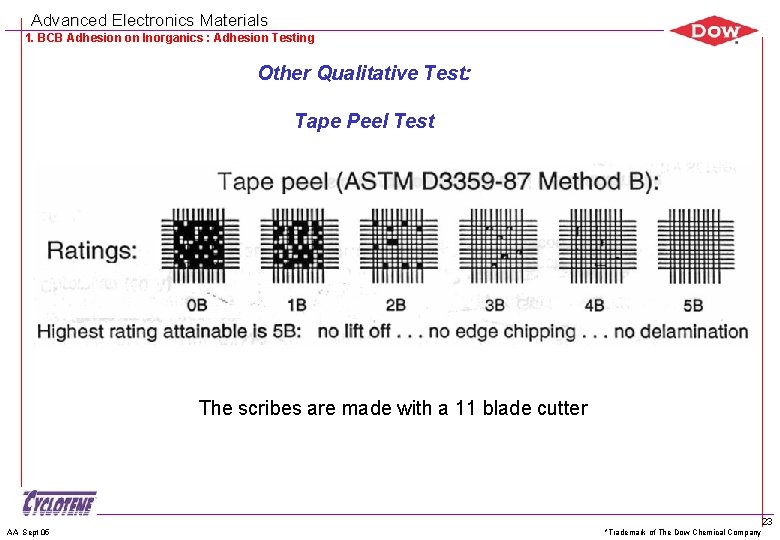
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Other Qualitative Test: Tape Peel Test The scribes are made with a 11 blade cutter AA Sept 05 *Trademark of The Dow Chemical Company 23

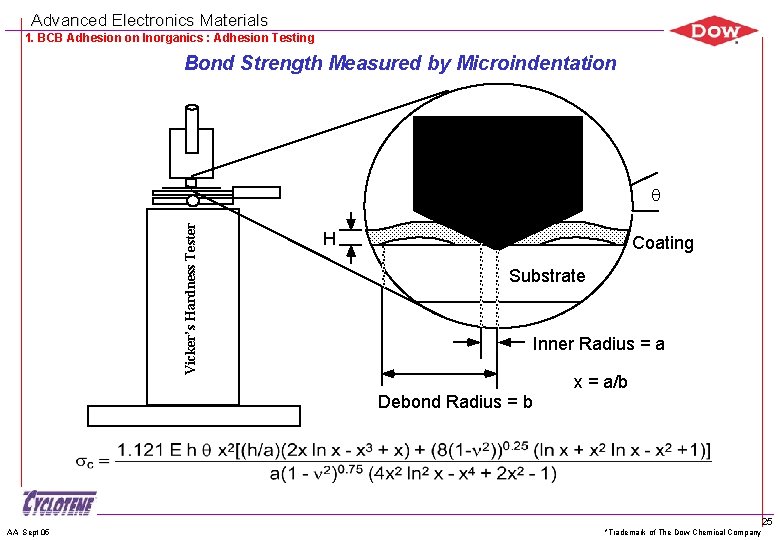
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Bond Strength Measured by Microindentation AA Sept 05 *Trademark of The Dow Chemical Company 24

Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Bond Strength Measured by Microindentation Vicker’s Hardness Tester q H Coating Substrate Inner Radius = a x = a/b Debond Radius = b AA Sept 05 *Trademark of The Dow Chemical Company 25

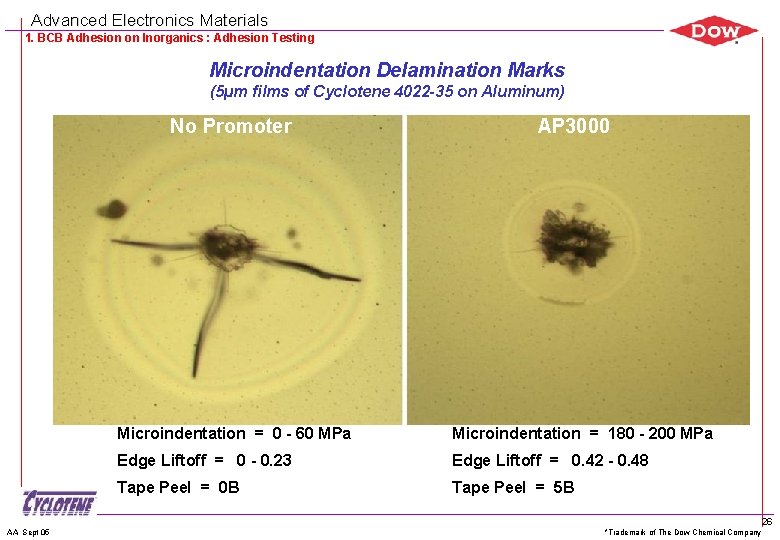
Advanced Electronics Materials 1. BCB Adhesion on Inorganics : Adhesion Testing Microindentation Delamination Marks (5µm films of Cyclotene 4022 -35 on Aluminum) No Promoter AA Sept 05 AP 3000 Microindentation = 0 - 60 MPa Microindentation = 180 - 200 MPa Edge Liftoff = 0 - 0. 23 Edge Liftoff = 0. 42 - 0. 48 Tape Peel = 0 B Tape Peel = 5 B *Trademark of The Dow Chemical Company 26

Advanced Electronics Materials 2. Metal Adhesion on BCB Metals Deposition on BCB Ø Metal on BCB without tie layer : Aluminum Ø Metal on BCB with metallic tie layer : Cu, Au, Pt, Ø Most used metal tie layers : Ti, Ti/W (10/90), Cr, Ta, V. . . Ø With tie layers , the metal is strongly bonded onto BCB. Ø Final adhesion depends on various process parameters of which the in-situ surface “activation” using Ar plasma is the most critical. Ø Too heavy Ar sputtering however will “carbonize” the polymer surface, causing lack of adhesion and surface conductivity. Ø Depending on tool and conditions, the sputtered tie metal can also penetrate into the BCB layer as deep as 200 nm. Ø The following pages show the influence on Ar soft-etch on the metal film growth and structure on cured BCB. AA Sept 05 *Trademark of The Dow Chemical Company 27

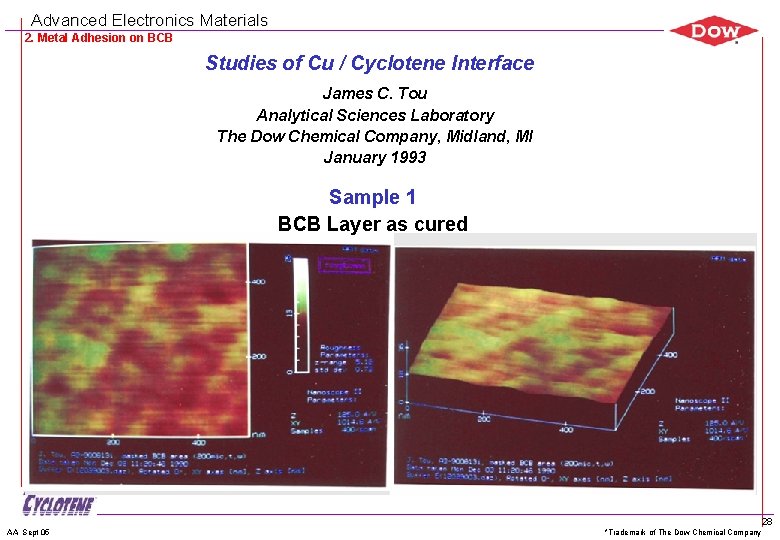
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface James C. Tou Analytical Sciences Laboratory The Dow Chemical Company, Midland, MI January 1993 Sample 1 BCB Layer as cured AA Sept 05 *Trademark of The Dow Chemical Company 28

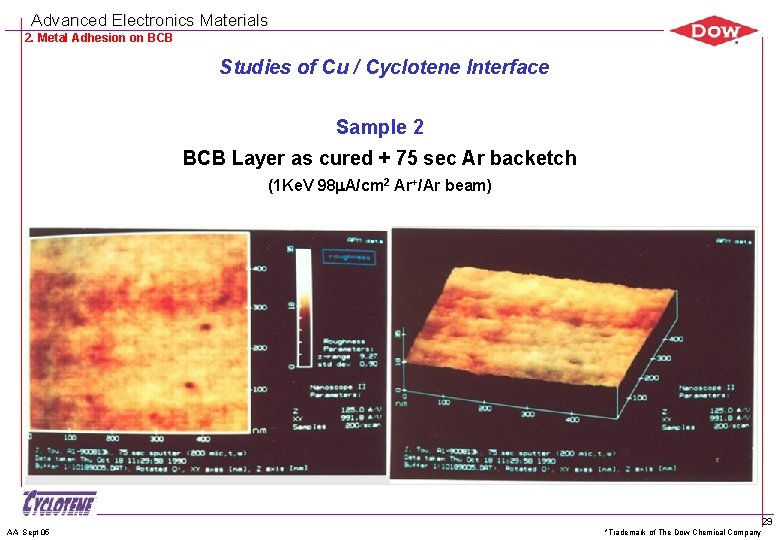
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Sample 2 BCB Layer as cured + 75 sec Ar backetch (1 Ke. V 98 m. A/cm 2 Ar+/Ar beam) AA Sept 05 *Trademark of The Dow Chemical Company 29

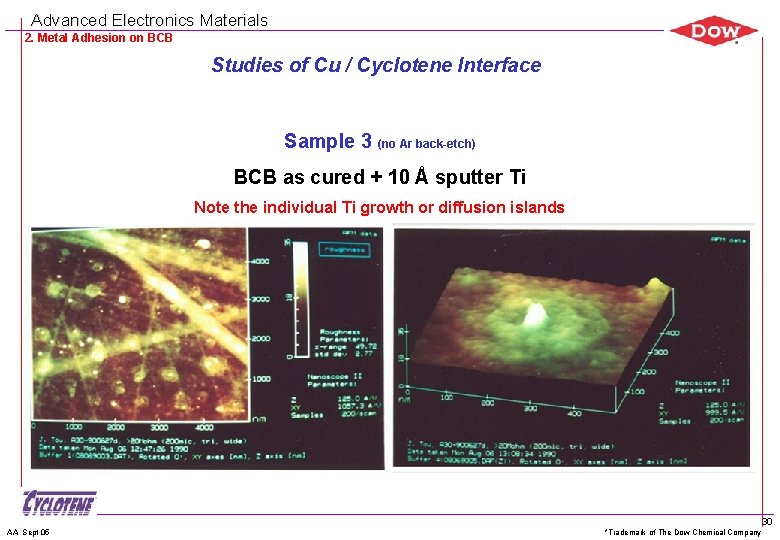
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Sample 3 (no Ar back-etch) BCB as cured + 10 Å sputter Ti Note the individual Ti growth or diffusion islands AA Sept 05 *Trademark of The Dow Chemical Company 30

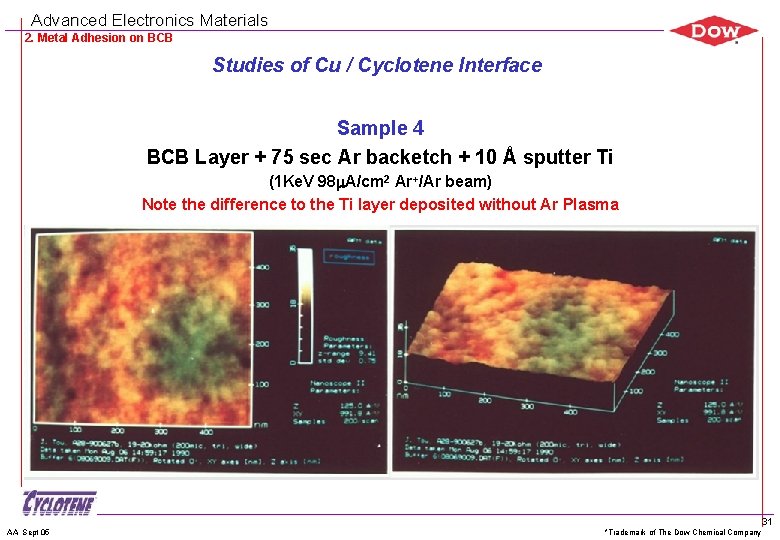
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Sample 4 BCB Layer + 75 sec Ar backetch + 10 Å sputter Ti (1 Ke. V 98 m. A/cm 2 Ar+/Ar beam) Note the difference to the Ti layer deposited without Ar Plasma AA Sept 05 *Trademark of The Dow Chemical Company 31

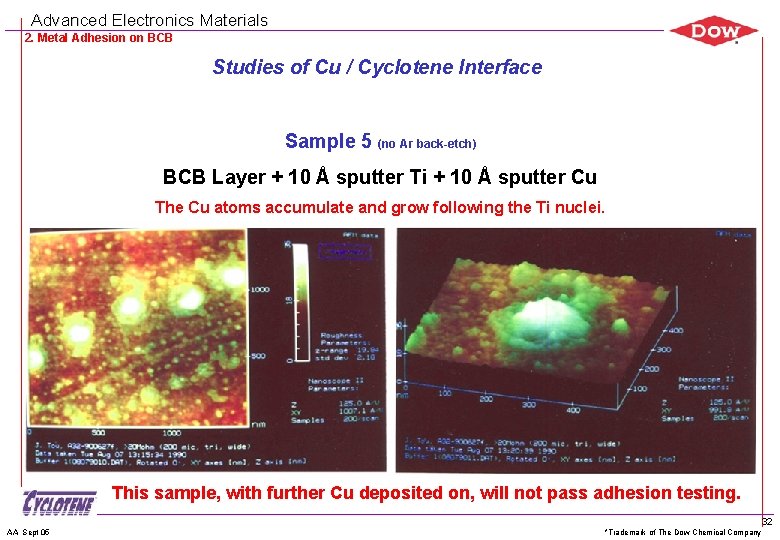
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Sample 5 (no Ar back-etch) BCB Layer + 10 Å sputter Ti + 10 Å sputter Cu The Cu atoms accumulate and grow following the Ti nuclei. This sample, with further Cu deposited on, will not pass adhesion testing. AA Sept 05 *Trademark of The Dow Chemical Company 32

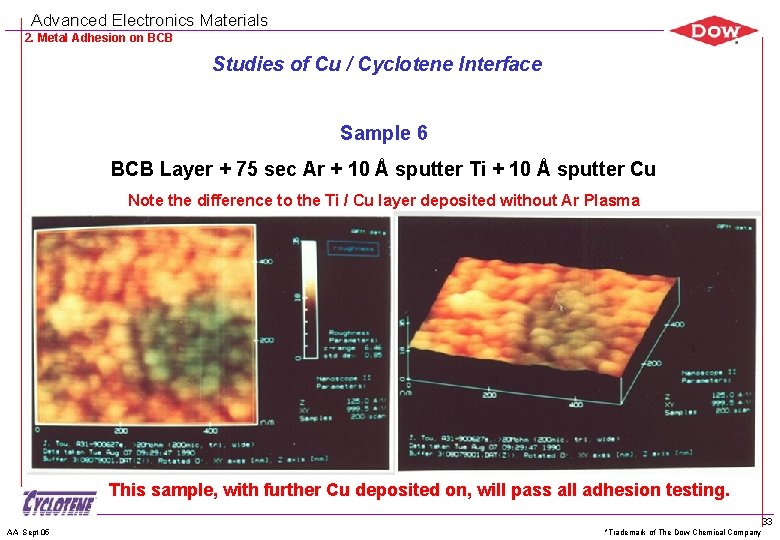
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Sample 6 BCB Layer + 75 sec Ar + 10 Å sputter Ti + 10 Å sputter Cu Note the difference to the Ti / Cu layer deposited without Ar Plasma This sample, with further Cu deposited on, will pass all adhesion testing. AA Sept 05 *Trademark of The Dow Chemical Company 33

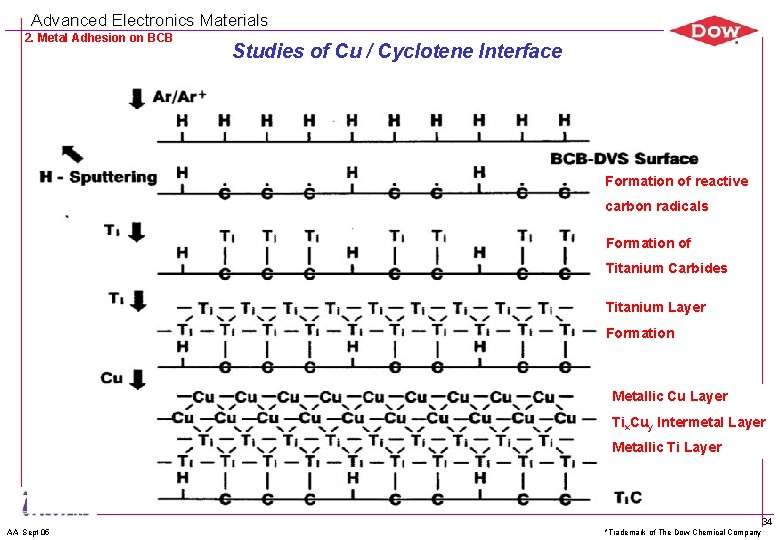
Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Formation of reactive carbon radicals Formation of Titanium Carbides Titanium Layer Formation Metallic Cu Layer Tix. Cuy Intermetal Layer Metallic Ti Layer AA Sept 05 *Trademark of The Dow Chemical Company 34

Advanced Electronics Materials 2. Metal Adhesion on BCB Studies of Cu / Cyclotene Interface Ø This study looked at the influence of Ar plasma and Ti layer on Cu adhesion to BCB. Ø No plasma sputter conditions (Ar, N 2, O 2) could be found to achieve good Cu adhesion without tie layer. Ø Without Ar plasma soft etch and without Ti tie layer, the Cu layer will not adhere to the BCB film. Ø The Ar plasma plays a role in the way the metal atoms nucleate and form a film on the polymer layer. Ø The dominant mechanism for good adhesion is the formation of metal-organic carbide bonds between the transition metal and the organic polymer carbon (Ti-C, W-C, Cr-C, ) Ø This is also true for Au (Ar/Ti/Au). Ø Aluminium does not need a tie layer, but Ar softetch is necessary for adhesion on BCB. Ø It is important to control Ar process to avoid over heating the BCB and change the % cure when processing multilayers (70 – 85% softcured layers needed for inter-BCB adhesion). AA Sept 05 *Trademark of The Dow Chemical Company 35

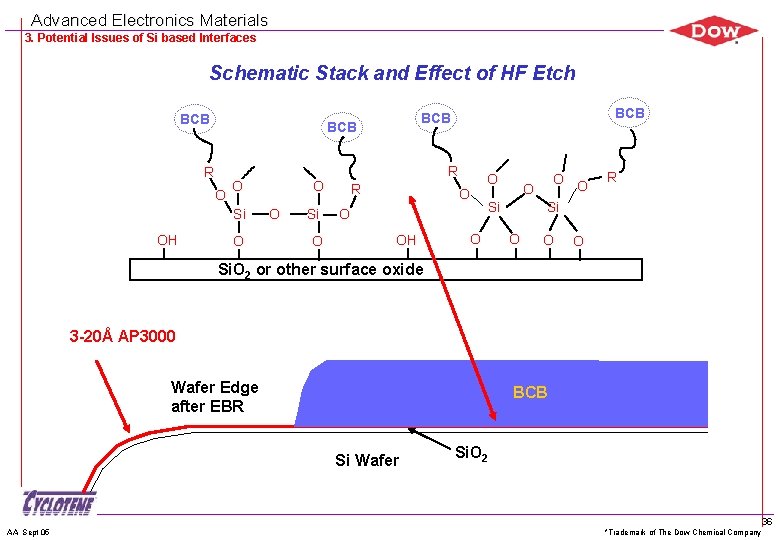
Advanced Electronics Materials 3. Potential Issues of Si based Interfaces Schematic Stack and Effect of HF Etch BCB R O Si OH O R O O O Si O BCB R O O Si O OH O O R Si O O O Si. O 2 or other surface oxide 3 -20Å AP 3000 Wafer Edge after EBR BCB Si Wafer AA Sept 05 Si. O 2 *Trademark of The Dow Chemical Company 36

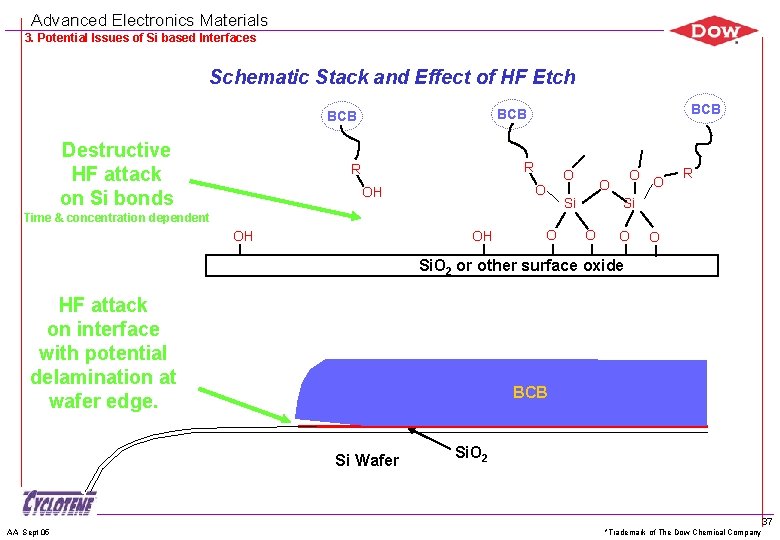
Advanced Electronics Materials 3. Potential Issues of Si based Interfaces Schematic Stack and Effect of HF Etch Destructive HF attack on Si bonds BCB BCB R R O O OH O O Si O R Si Time & concentration dependent OH OH O O Si. O 2 or other surface oxide HF attack on interface with potential delamination at wafer edge. BCB Si Wafer AA Sept 05 Si. O 2 *Trademark of The Dow Chemical Company 37