A Computational TDDFT Study on Intramolecular Charge Transfer
- Slides: 13

A Computational TDDFT Study on Intramolecular Charge Transfer in Di-tert-butylaminobenzonitriles and 2, 4, 6 -Tricyanoanilines Takashige Fujiwara Department of Chemistry and Biochemistry The Ohio State University Marek Z. Zgierski National Research Council of Canada 69 th ISMS FC 10 Urbana-Champaign, IL E-mail: fujiwara@physics. osu. edu

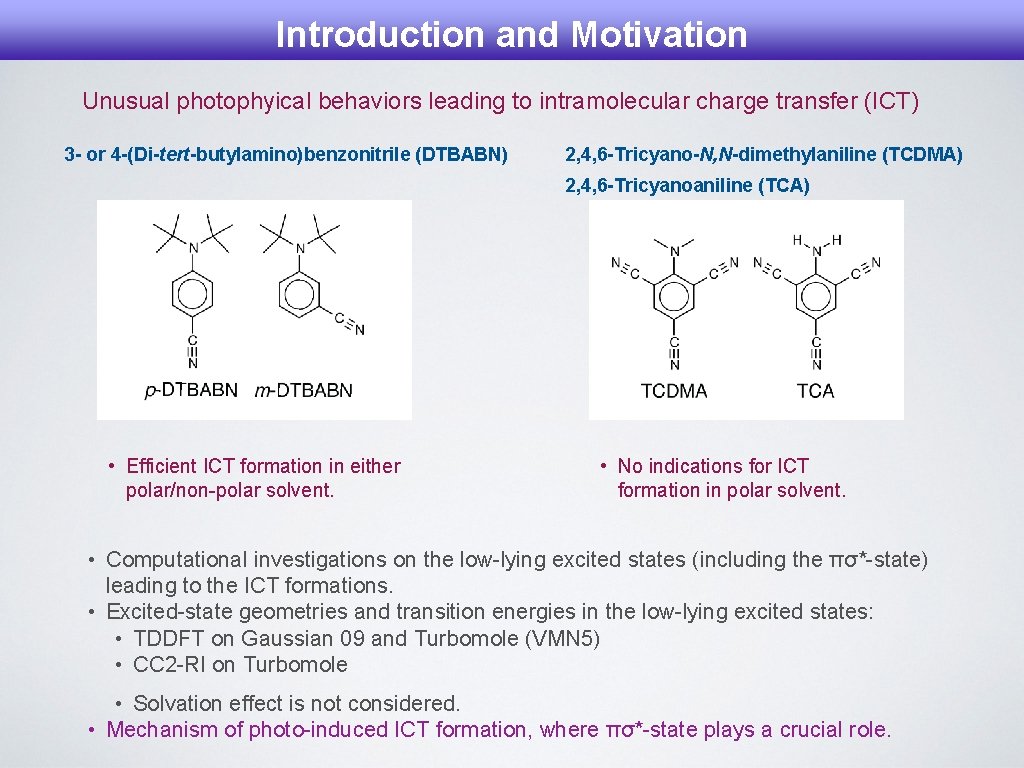
Introduction and Motivation Unusual photophyical behaviors leading to intramolecular charge transfer (ICT) 3 - or 4 -(Di-tert-butylamino)benzonitrile (DTBABN) 2, 4, 6 -Tricyano-N, N-dimethylaniline (TCDMA) 2, 4, 6 -Tricyanoaniline (TCA) • Efficient ICT formation in either polar/non-polar solvent. • No indications for ICT formation in polar solvent. • Computational investigations on the low-lying excited states (including the πσ*-state) leading to the ICT formations. • Excited-state geometries and transition energies in the low-lying excited states: • TDDFT on Gaussian 09 and Turbomole (VMN 5) • CC 2 -RI on Turbomole • Solvation effect is not considered. • Mechanism of photo-induced ICT formation, where πσ*-state plays a crucial role.

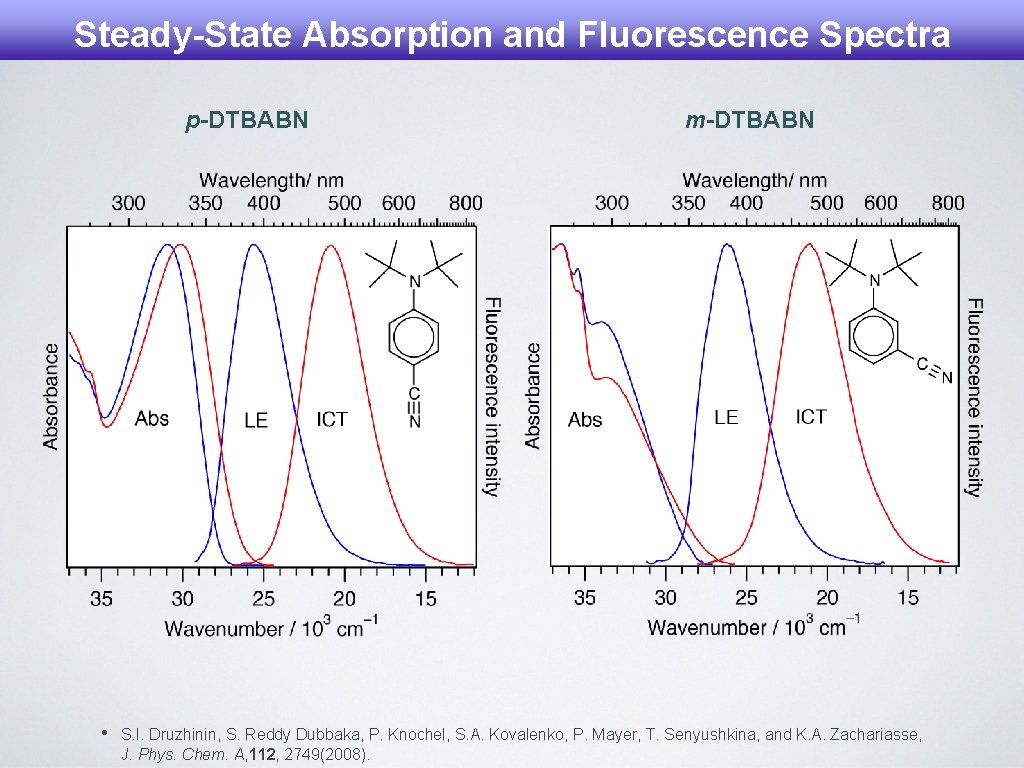
Steady-State Absorption and Fluorescence Spectra p-DTBABN • m-DTBABN S. I. Druzhinin, S. Reddy Dubbaka, P. Knochel, S. A. Kovalenko, P. Mayer, T. Senyushkina, and K. A. Zachariasse, J. Phys. Chem. A, 112, 2749(2008).

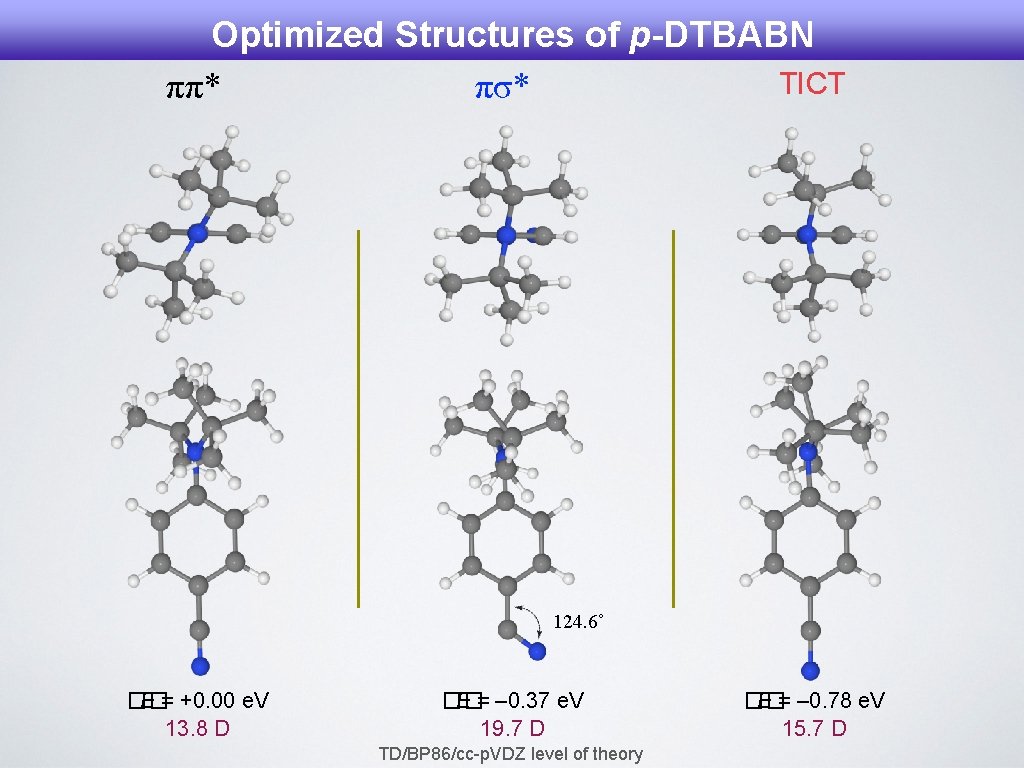
Optimized Structures of p-DTBABN TICT ππ* πσ* 124. 6˚ �� E = +0. 00 e. V 13. 8 D �� E = – 0. 37 e. V 19. 7 D TD/BP 86/cc-p. VDZ level of theory �� E = – 0. 78 e. V 15. 7 D

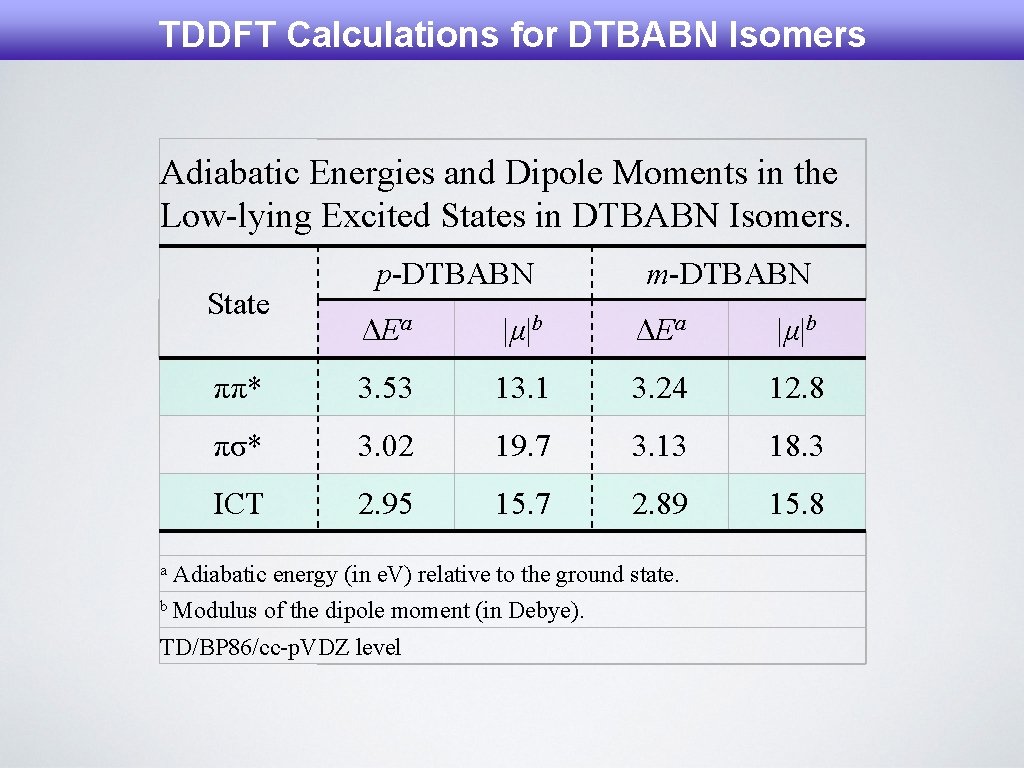
TDDFT Calculations for DTBABN Isomers Adiabatic Energies and Dipole Moments in the Low-lying Excited States in DTBABN Isomers. State a p-DTBABN m-DTBABN ΔEa |μ|b ππ* 3. 53 13. 1 3. 24 12. 8 πσ* 3. 02 19. 7 3. 13 18. 3 ICT 2. 95 15. 7 2. 89 15. 8 Adiabatic energy (in e. V) relative to the ground state. b Modulus of the dipole moment (in Debye). TD/BP 86/cc-p. VDZ level

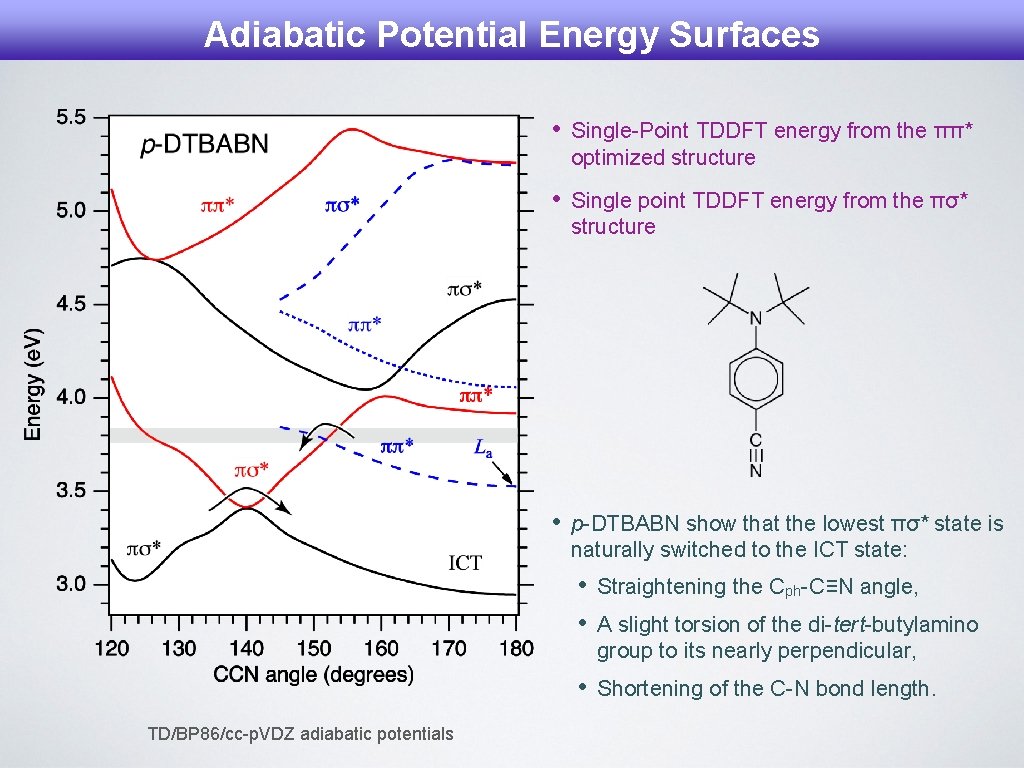
Adiabatic Potential Energy Surfaces TD/BP 86/cc-p. VDZ adiabatic potentials • Single-Point TDDFT energy from the ππ* optimized structure • Single point TDDFT energy from the πσ* structure • p-DTBABN show that the lowest πσ* state is naturally switched to the ICT state: • • Straightening the Cph-C≡N angle, • Shortening of the C-N bond length. A slight torsion of the di-tert-butylamino group to its nearly perpendicular,

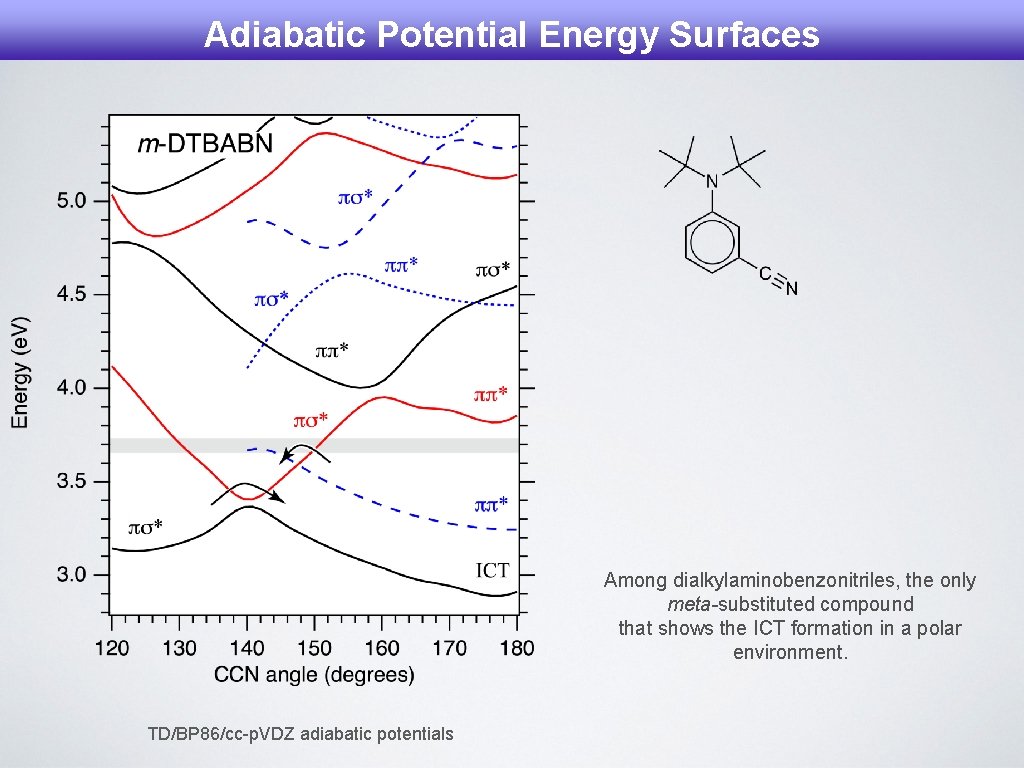
Adiabatic Potential Energy Surfaces Among dialkylaminobenzonitriles, the only meta-substituted compound that shows the ICT formation in a polar environment. TD/BP 86/cc-p. VDZ adiabatic potentials

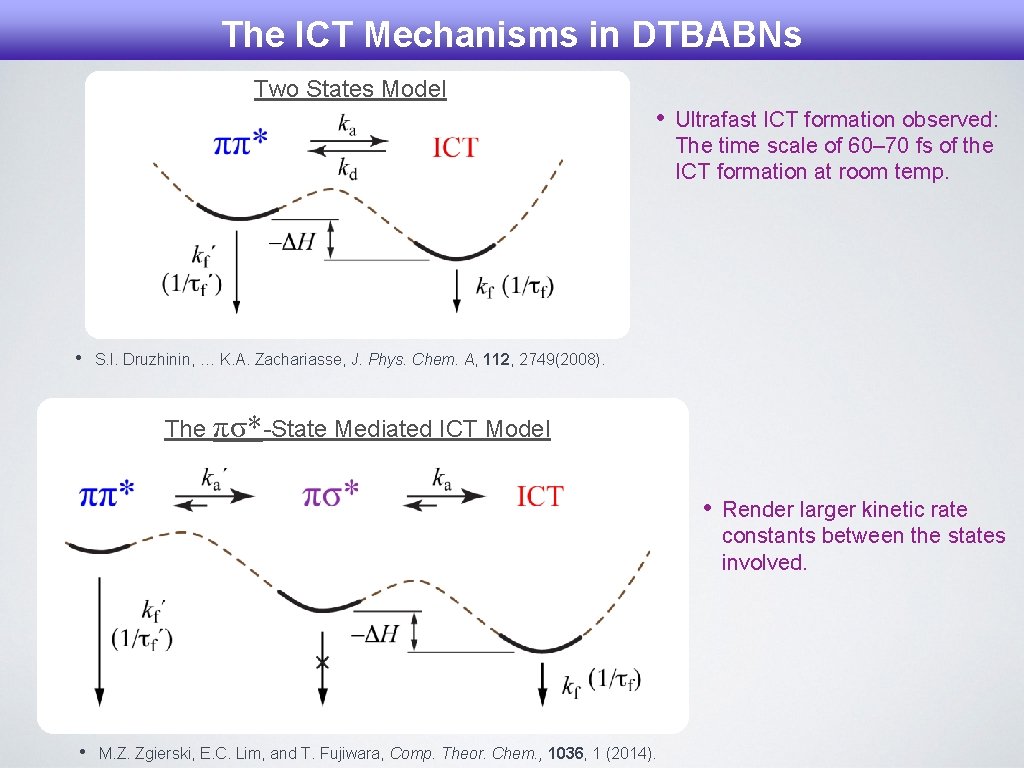
The ICT Mechanisms in DTBABNs Two States Model • • Ultrafast ICT formation observed: The time scale of 60– 70 fs of the ICT formation at room temp. S. I. Druzhinin, … K. A. Zachariasse, J. Phys. Chem. A, 112, 2749(2008). The πσ*-State Mediated ICT Model • • M. Z. Zgierski, E. C. Lim, and T. Fujiwara, Comp. Theor. Chem. , 1036, 1 (2014). Render larger kinetic rate constants between the states involved.

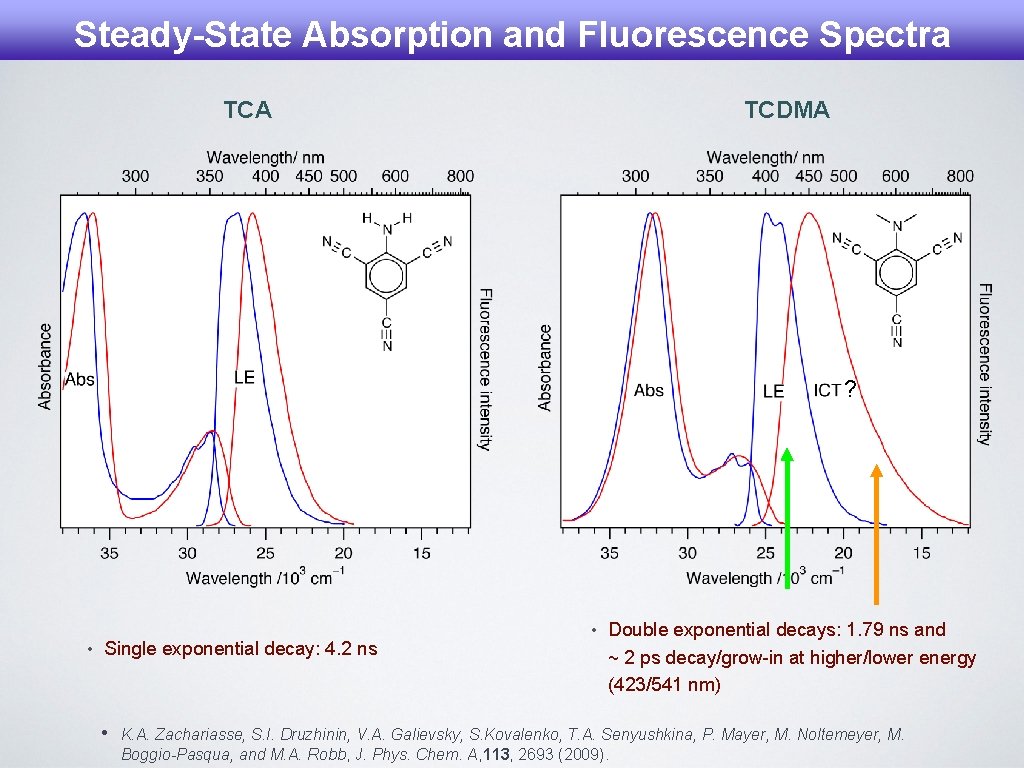
Steady-State Absorption and Fluorescence Spectra TCA TCDMA ? • Single exponential decay: 4. 2 ns • • Double exponential decays: 1. 79 ns and ~ 2 ps decay/grow-in at higher/lower energy (423/541 nm) K. A. Zachariasse, S. I. Druzhinin, V. A. Galievsky, S. Kovalenko, T. A. Senyushkina, P. Mayer, M. Noltemeyer, M. Boggio-Pasqua, and M. A. Robb, J. Phys. Chem. A, 113, 2693 (2009).

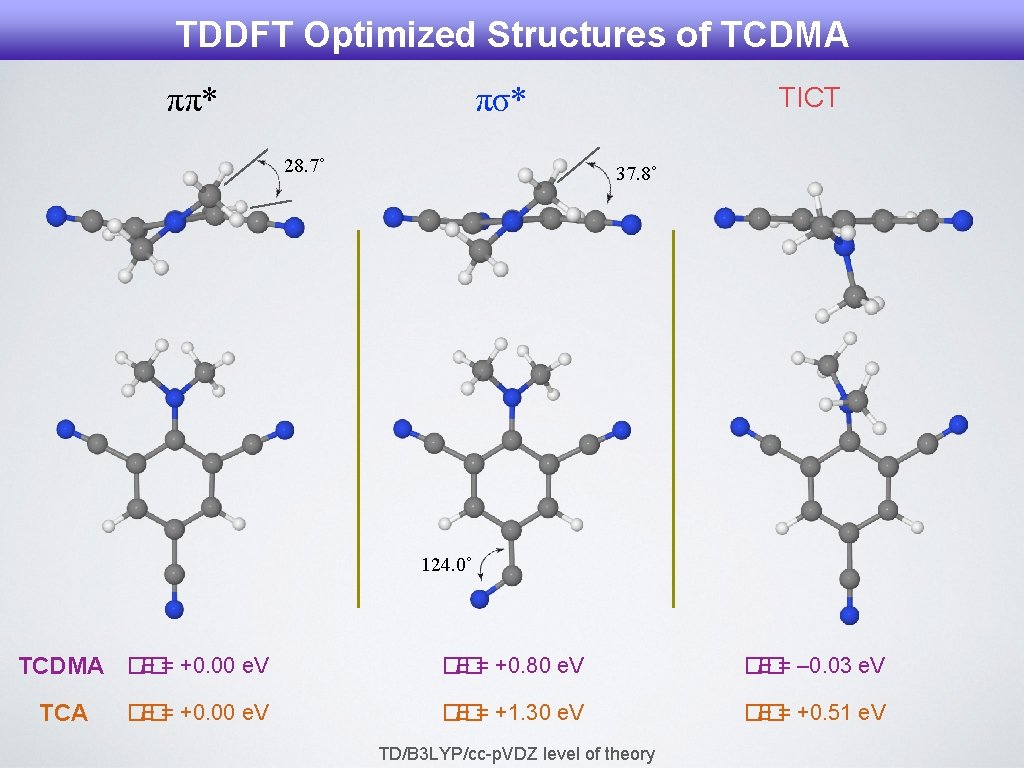
TDDFT Optimized Structures of TCDMA ππ* πσ* 28. 7˚ TICT 37. 8˚ 124. 0˚ E = +0. 00 e. V TCDMA �� TCA �� E = +0. 00 e. V �� E = +0. 80 e. V �� E = – 0. 03 e. V �� E = +1. 30 e. V �� E = +0. 51 e. V TD/B 3 LYP/cc-p. VDZ level of theory

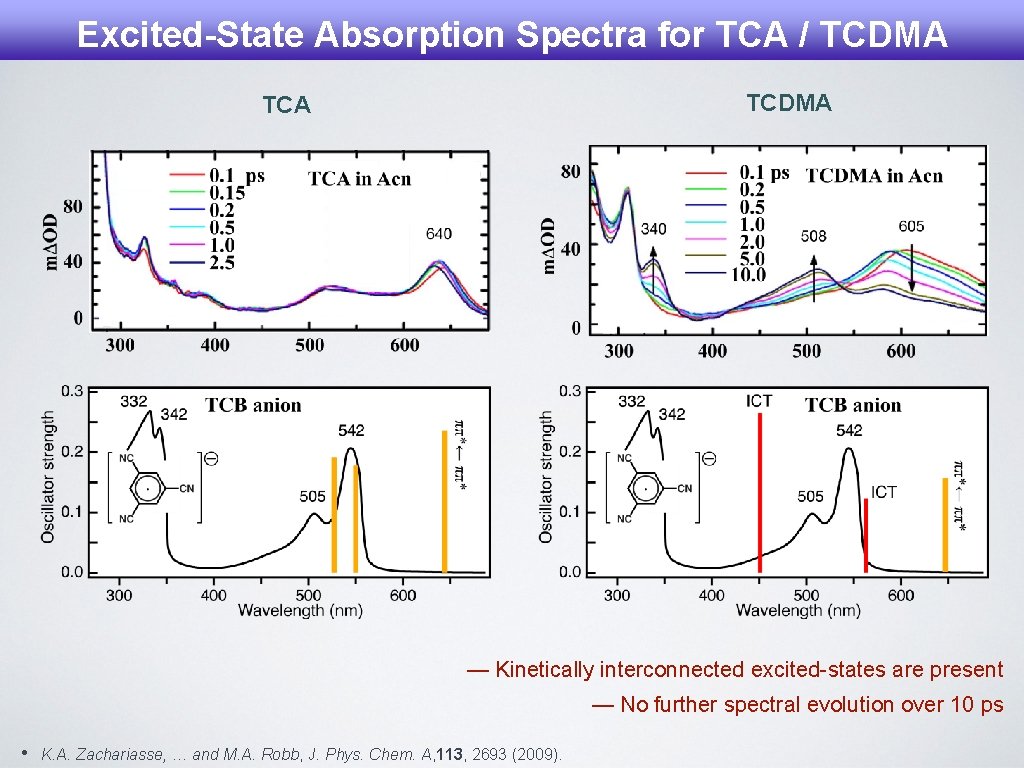
Excited-State Absorption Spectra for TCA / TCDMA TCA — Kinetically interconnected excited-states are present — No further spectral evolution over 10 ps • K. A. Zachariasse, … and M. A. Robb, J. Phys. Chem. A, 113, 2693 (2009).

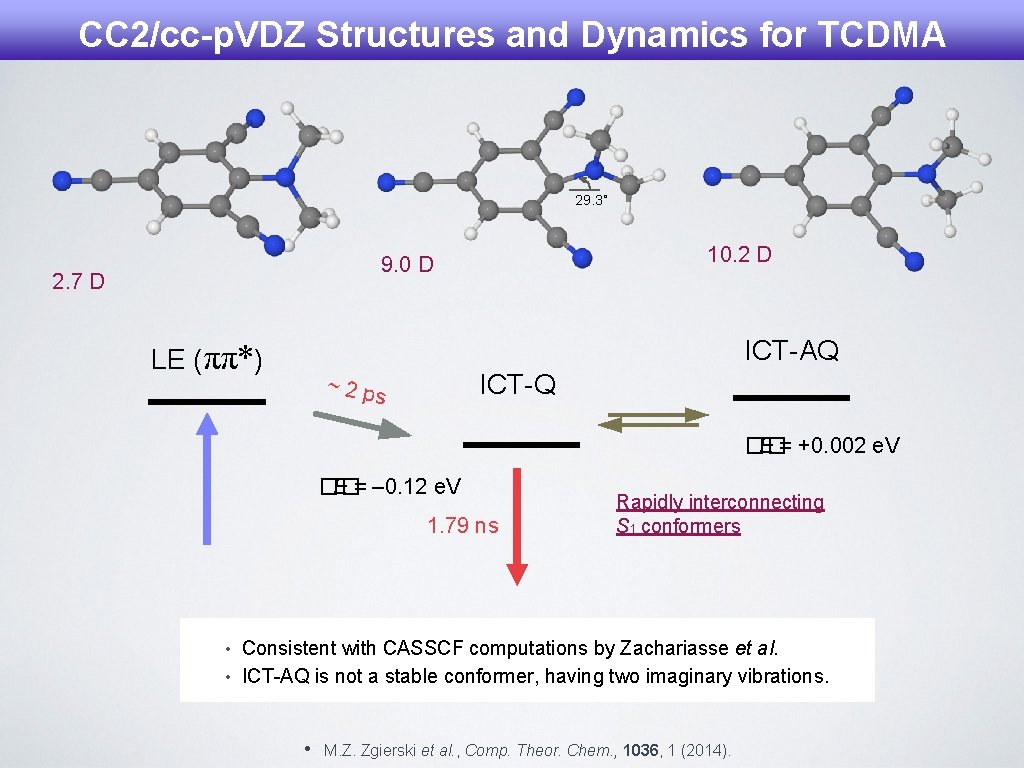
CC 2/cc-p. VDZ Structures and Dynamics for TCDMA 29. 3˚ 10. 2 D 9. 0 D 2. 7 D ICT-AQ LE (ππ*) ICT-Q ~ 2 ps �� E = +0. 002 e. V �� E = – 0. 12 e. V 1. 79 ns Rapidly interconnecting S 1 conformers • Consistent with CASSCF computations by Zachariasse et al. • ICT-AQ is not a stable conformer, having two imaginary vibrations. • M. Z. Zgierski et al. , Comp. Theor. Chem. , 1036, 1 (2014).

Summary and Acknowledgment TDDFT (or CC 2) computations were performed on the low-lying excited states of di-tert-butylaminobenzonitriles and tricyanoanilines that exhibit unusual photophysical behaviors leading to the ICT formation. Ultrafast ICT formation in p-DTBABN and m-DTBABN is due to the sequential mechanism of ππ*→ πσ*→ ICT, involving conical intersections among the closely-lying excited-states. For TCDMA, the presence of a TICT state that lies below the initially photoexcited ππ* state, responsible for the ultrafast dynamics observed in the excited-state absorption in acetonitrile. In both cases for TCDMA and TCA, the πσ* state locates significantly higher in energy than the ππ* state, thus precluding the πσ*→ ICT formation. Collaborator Dr. Edward C. Lim, The University of Akron, U. S. A Support Ohio Supercomputer Center