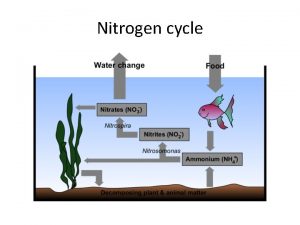
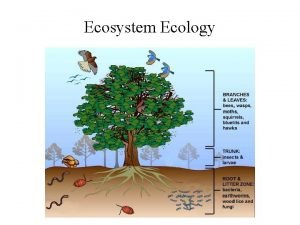
3 NITROGEN CYCLE SOIL 5813 SoilPlant Nutrient Cycling







![◦ p. H = p. Ka + log [(base)/(acid)] ◦ p. Kw = p. ◦ p. H = p. Ka + log [(base)/(acid)] ◦ p. Kw = p.](https://slidetodoc.com/presentation_image_h/c957fac9948ed00881c28c716fdf1cd6/image-28.jpg)









- Slides: 59

3. NITROGEN CYCLE SOIL 5813 Soil-Plant Nutrient Cycling and Environmental Quality Department of Plant and Soil Sciences Oklahoma State University Stillwater, OK 74078 email: wrr@mail. pss. okstate. edu Tel: (405) 744 -6414

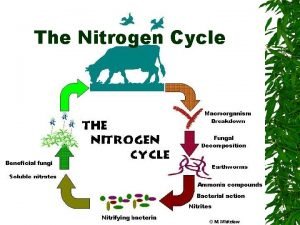
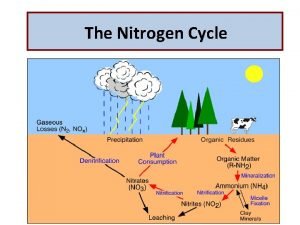
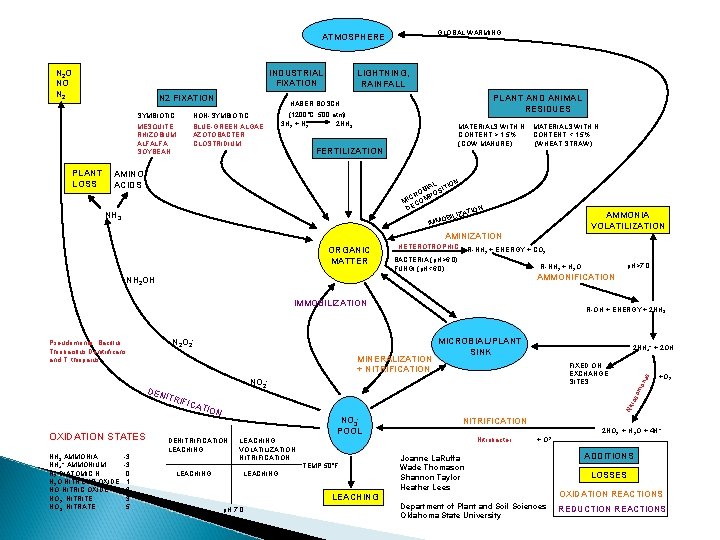
GLOBAL WARMING ATMOSPHERE N 2 O NO N 2 PLANT LOSS INDUSTRIAL FIXATION N 2 FIXATION LIGHTNING, RAINFALL PLANT AND ANIMAL RESIDUES HABER BOSCH SYMBIOTIC NON-SYMBIOTIC MESQUITE RHIZOBIUM ALFALFA SOYBEAN BLUE-GREEN ALGAE AZOTOBACTER CLOSTRIDIUM (1200°C, 500 atm) 3 H 2 + N 2 2 NH 3 MATERIALS WITH N CONTENT > 1. 5% (COW MANURE) FERTILIZATION AMINO ACIDS MATERIALS WITH N CONTENT < 1. 5% (WHEAT STRAW) N IAL ITIO OB POS R C MI COM DE N ATIO BILIZ O M IM NH 3 AMMONIA VOLATILIZATION AMINIZATION ORGANIC MATTER HETEROTROPHIC R-NH 2 + ENERGY + CO 2 BACTERIA (p. H>6. 0) FUNGI (p. H<6. 0) AMMONIFICATION NH 2 OH IMMOBILIZATION -3 -3 0 1 2 3 5 FIXED ON EXCHANGE SITES NO 2 - ros o NO 3 POOL DENITRIFICATION LEACHING +O 2 Nit TION NH 3 AMMONIA NH 4+ AMMONIUM N 2 DIATOMIC N N 2 O NITROUS OXIDE NO NITRIC OXIDE NO 2 - NITRITE NO 3 - NITRATE 2 NH 4+ + 2 OH- s MINERALIZATION + NITRIFICATION MICROBIAL/PLANT SINK na N 2 O 2 - DEN ITRI FICA OXIDATION STATES R-OH + ENERGY + 2 NH 3 mo Pseudomonas, Bacillus, Thiobacillus Denitrificans, and T. thioparus p. H>7. 0 R-NH 2 + H 2 O LEACHING VOLATILIZATION NITRIFICATION LEACHING TEMP 50°F LEACHING p. H 7. 0 NITRIFICATION Nitrobacter + O 2 Joanne La. Ruffa Wade Thomason Shannon Taylor Heather Lees Department of Plant and Soil Sciences Oklahoma State University 2 NO 2 - + H 2 O + 4 H+ ADDITIONS LOSSES OXIDATION REACTIONS REDUCTION REACTIONS

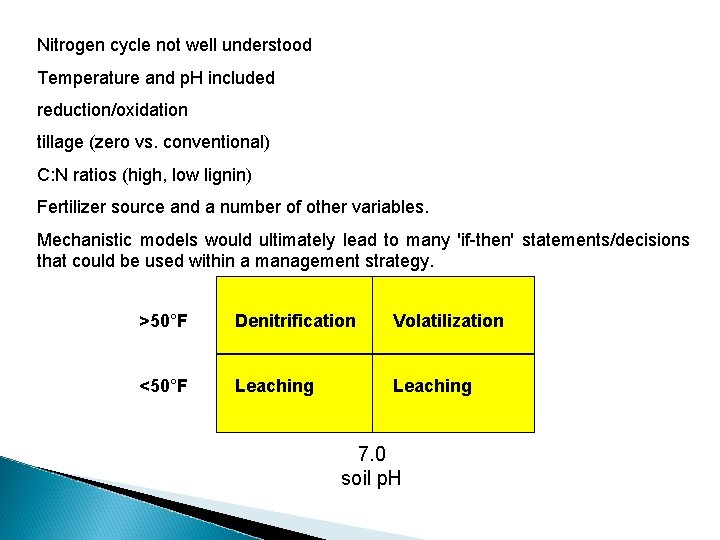
Nitrogen cycle not well understood Temperature and p. H included reduction/oxidation tillage (zero vs. conventional) C: N ratios (high, low lignin) Fertilizer source and a number of other variables. Mechanistic models would ultimately lead to many 'if-then' statements/decisions that could be used within a management strategy. >50°F Denitrification Volatilization <50°F Leaching 7. 0 soil p. H

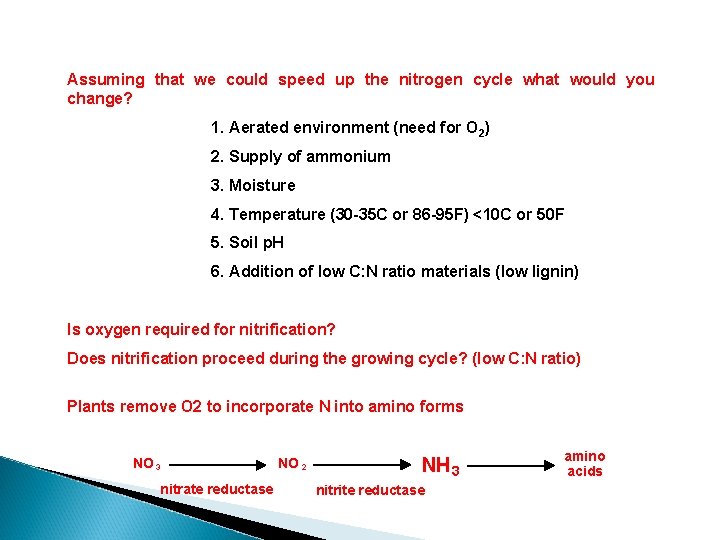
Assuming that we could speed up the nitrogen cycle what would you change? 1. Aerated environment (need for O 2) 2. Supply of ammonium 3. Moisture 4. Temperature (30 -35 C or 86 -95 F) <10 C or 50 F 5. Soil p. H 6. Addition of low C: N ratio materials (low lignin) Is oxygen required for nitrification? Does nitrification proceed during the growing cycle? (low C: N ratio) Plants remove O 2 to incorporate N into amino forms NO 3 nitrate reductase NO 2 NH 3 nitrite reductase amino acids

N recommendations 1. Yield goal (2 lb N/bu) a. Applies fertilization risk on the farmer b. Removes our inability to predict 'environment' (rainfall) 2. Soil test a. For every 1 ppm NO 3, N recommendation reduced by 2 lb. N/ac 3. Potential yield Nitrite accumulation? 1. high p. H 2. high NH 4 levels (NH 4 inhibits nitrobacter)

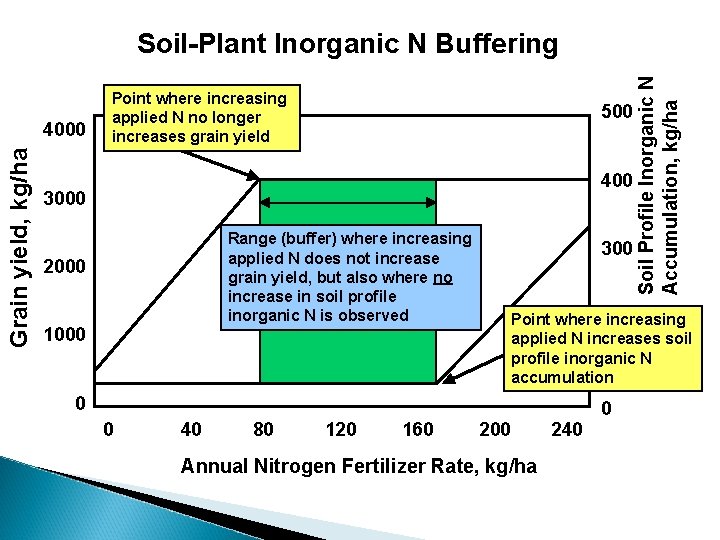
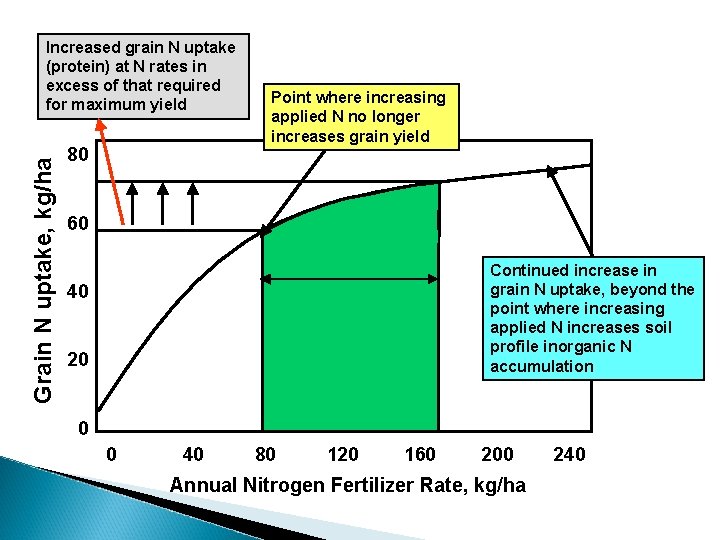
Inorganic Nitrogen Buffering Ability of the soil plant system to control the amount of inorganic N accumulation in the rooting profile when N fertilization rates exceed that required for maximum yield.

Grain yield, kg/ha 4000 Point where increasing applied N no longer increases grain yield 500 400 3000 Range (buffer) where increasing applied N does not increase grain yield, but also where no increase in soil profile inorganic N is observed 2000 1000 300 200 Point where increasing applied N increases soil profile inorganic N 100 accumulation 0 0 0 40 80 120 160 200 Soil Profile Inorganic N Accumulation, kg/ha Soil-Plant Inorganic N Buffering 240 Annual Nitrogen Fertilizer Rate, kg/ha

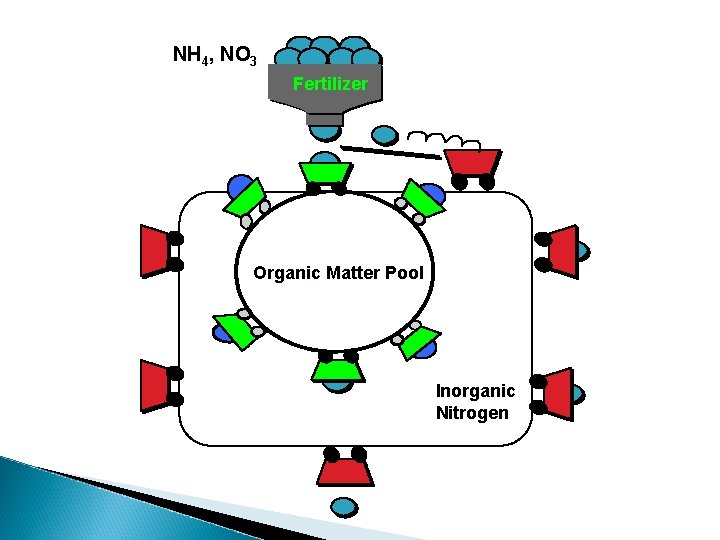
NH 4, NO 3 Fertilizer Organic Matter Pool Inorganic Nitrogen

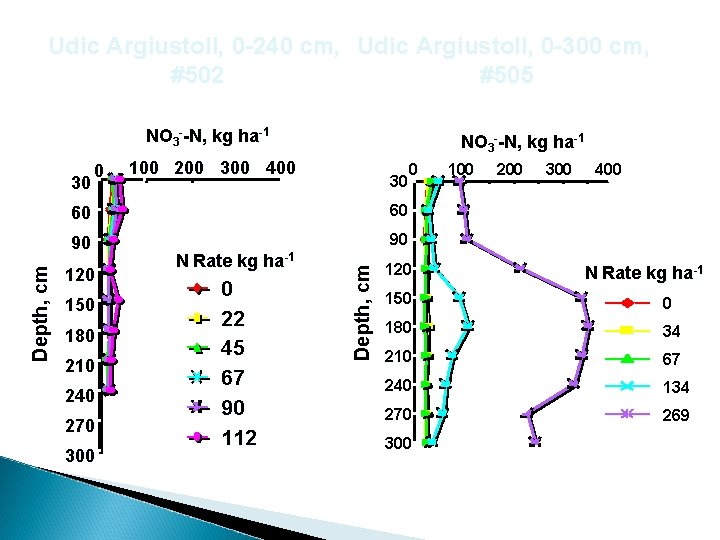
Udic Argiustoll, 0 -240 cm, Udic Argiustoll, 0 -300 cm, #502 #505 NO 3 --N, kg ha-1 100 200 300 400 30 60 60 90 90 120 150 180 210 240 270 300 N Rate kg ha-1 0 22 45 67 90 112 Depth, cm 30 0 NO 3 --N, kg ha-1 0 120 100 200 300 400 N Rate kg ha-1 150 0 180 34 210 67 240 134 270 269 300

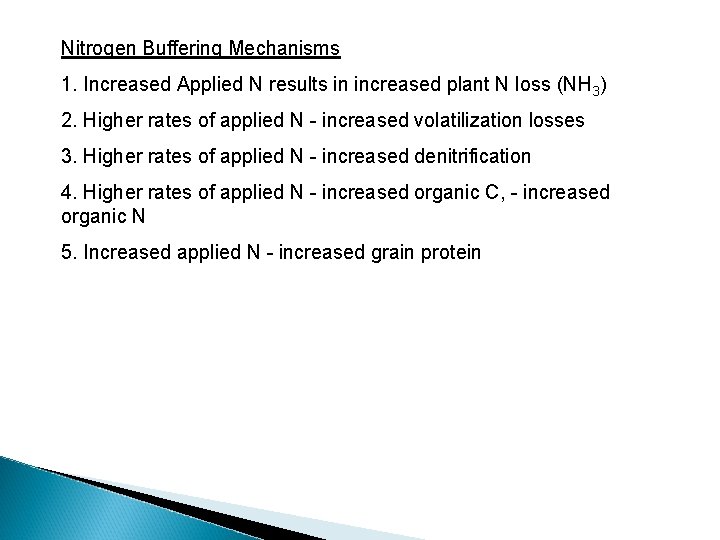
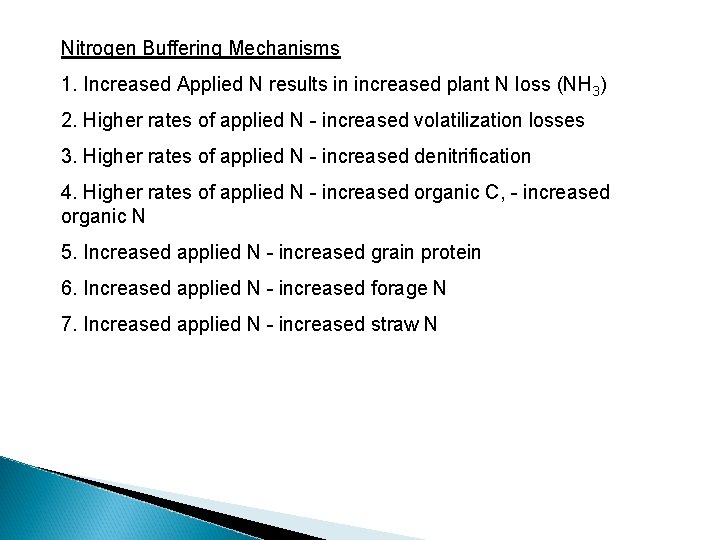
If the N rate required to detect soil profile NO 3 accumulation always exceeded that required for maximum yields, what biological mechanisms are present that cause leaching? excess N applied to be lost via other pathways prior to Nitrogen Buffering Mechanisms 1. Increased Applied N results in increased plant N loss (NH 3)

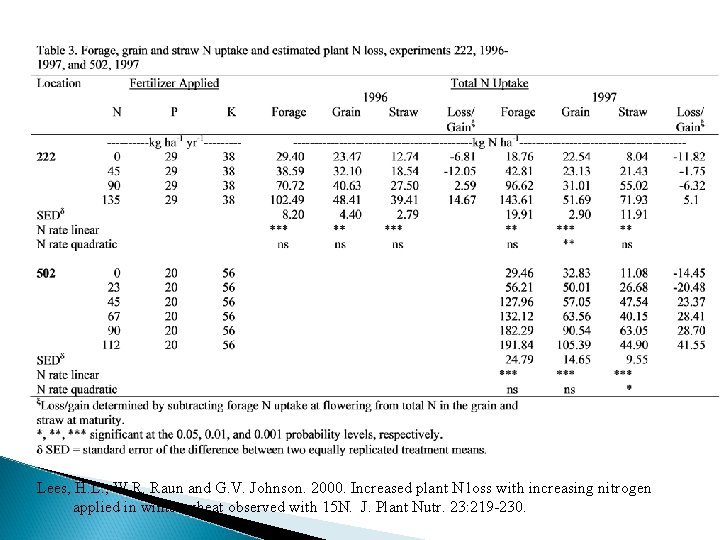
Lees, H. L. , W. R. Raun and G. V. Johnson. 2000. Increased plant N loss with increasing nitrogen applied in winter wheat observed with 15 N. J. Plant Nutr. 23: 219 -230.

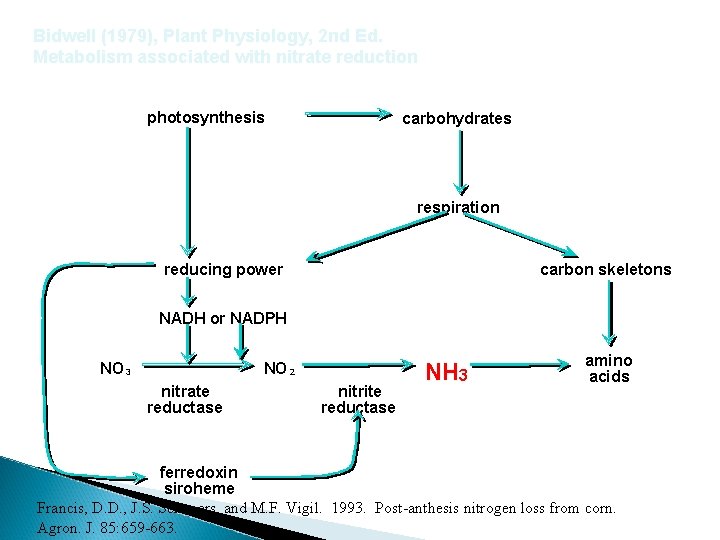
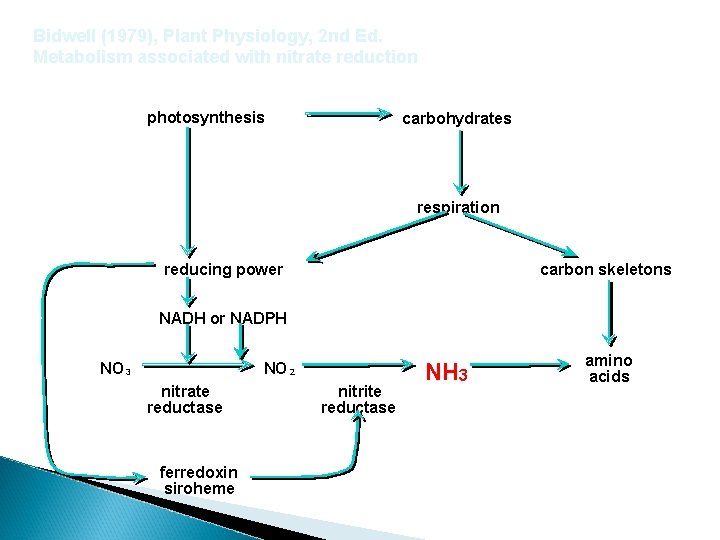
Bidwell (1979), Plant Physiology, 2 nd Ed. Metabolism associated with nitrate reduction photosynthesis carbohydrates respiration reducing power carbon skeletons NADH or NADPH NO 3 NO 2 nitrate reductase nitrite reductase NH 3 amino acids ferredoxin siroheme Francis, D. D. , J. S. Schepers, and M. F. Vigil. 1993. Post-anthesis nitrogen loss from corn. Agron. J. 85: 659 -663.

Nitrogen Buffering Mechanisms 1. Increased Applied N results in increased plant N loss (NH 3) 2. Higher rates of applied N - increased volatilization losses

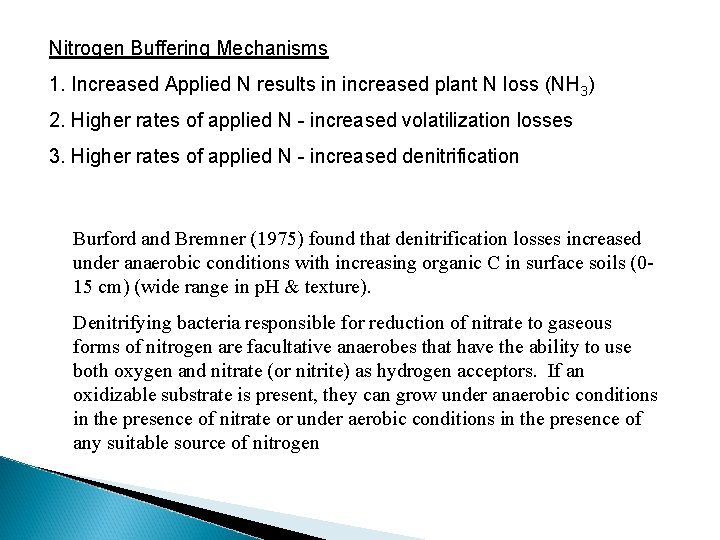
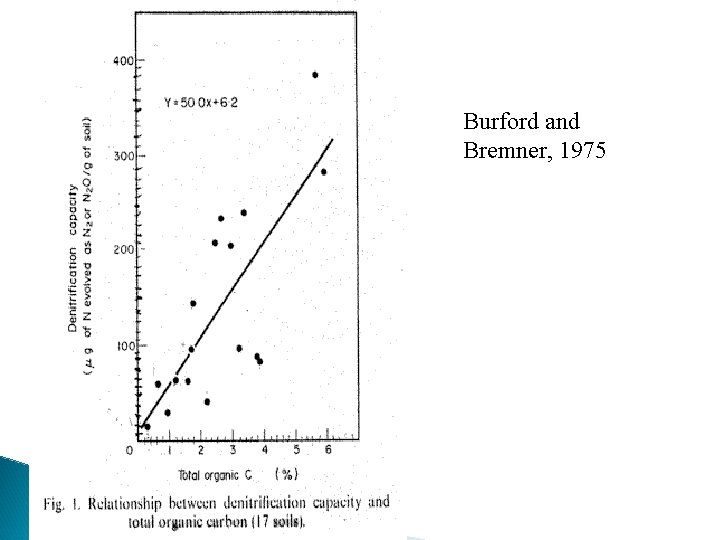
Nitrogen Buffering Mechanisms 1. Increased Applied N results in increased plant N loss (NH 3) 2. Higher rates of applied N - increased volatilization losses 3. Higher rates of applied N - increased denitrification Burford and Bremner (1975) found that denitrification losses increased under anaerobic conditions with increasing organic C in surface soils (015 cm) (wide range in p. H & texture). Denitrifying bacteria responsible for reduction of nitrate to gaseous forms of nitrogen are facultative anaerobes that have the ability to use both oxygen and nitrate (or nitrite) as hydrogen acceptors. If an oxidizable substrate is present, they can grow under anaerobic conditions in the presence of nitrate or under aerobic conditions in the presence of any suitable source of nitrogen

Burford and Bremner, 1975

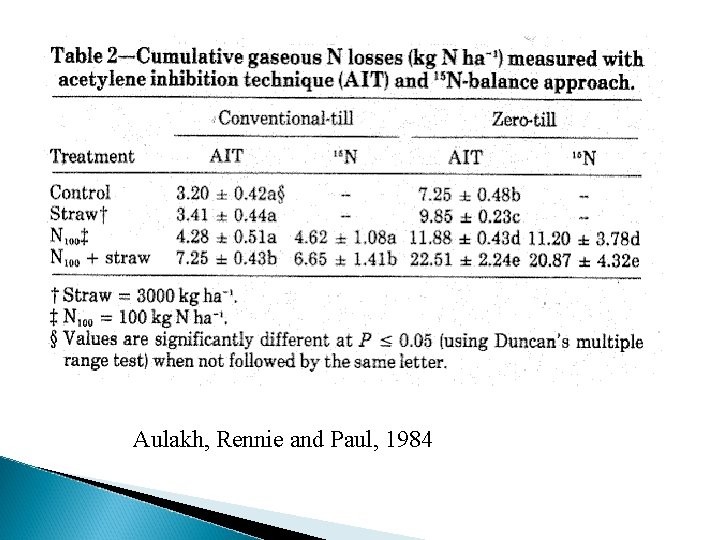
Aulakh, Rennie and Paul, 1984

Nitrogen Buffering Mechanisms 1. Increased Applied N results in increased plant N loss (NH 3) 2. Higher rates of applied N - increased volatilization losses 3. Higher rates of applied N - increased denitrification 4. Higher rates of applied N - increased organic C, - increased organic N

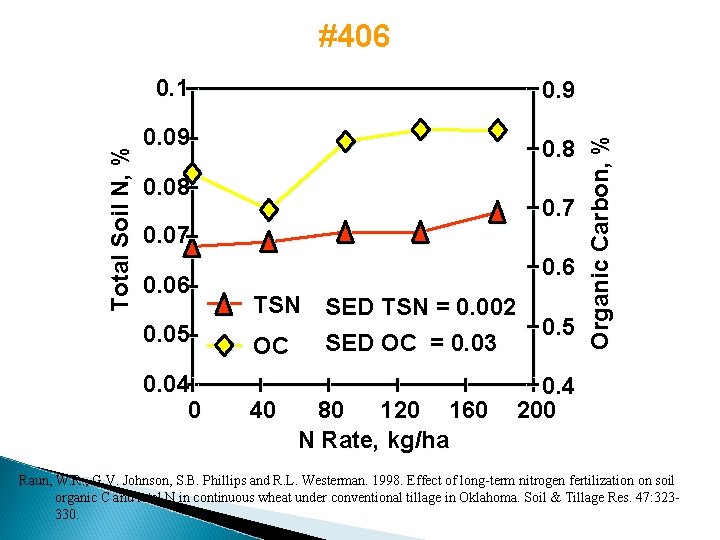
#406 0. 9 0. 09 0. 8 0. 08 0. 7 0. 06 0. 05 0. 04 0 0. 6 TSN SED TSN = 0. 002 SED OC = 0. 03 OC 40 80 120 160 N Rate, kg/ha 0. 5 Organic Carbon, % Total Soil N, % 0. 1 0. 4 200 Raun, W. R. , G. V. Johnson, S. B. Phillips and R. L. Westerman. 1998. Effect of long-term nitrogen fertilization on soil organic C and total N in continuous wheat under conventional tillage in Oklahoma. Soil & Tillage Res. 47: 323330.

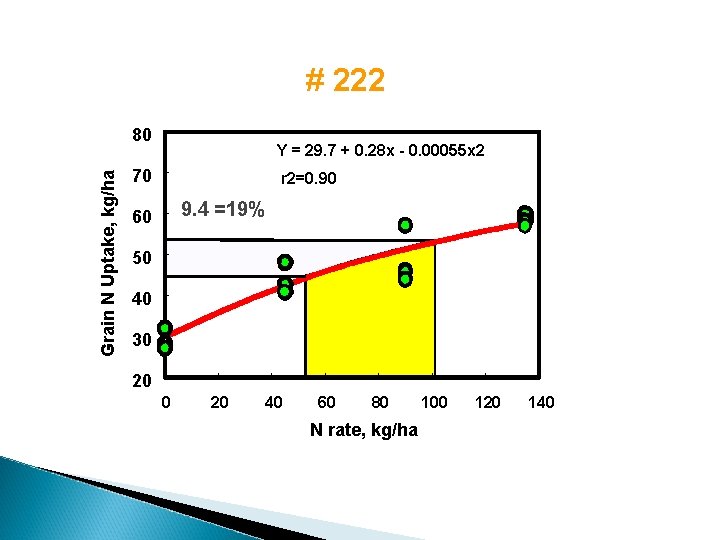
Nitrogen Buffering Mechanisms 1. Increased Applied N results in increased plant N loss (NH 3) 2. Higher rates of applied N - increased volatilization losses 3. Higher rates of applied N - increased denitrification 4. Higher rates of applied N - increased organic C, - increased organic N 5. Increased applied N - increased grain protein

Grain N uptake, kg/ha Increased grain N uptake (protein) at N rates in excess of that required for maximum yield 80 Point where increasing applied N no longer increases grain yield 60 Continued increase in grain N uptake, beyond the point where increasing applied N increases soil profile inorganic N accumulation 40 20 0 0 40 80 120 160 200 Annual Nitrogen Fertilizer Rate, kg/ha 240

# 222 Grain N Uptake, kg/ha 80 Y = 29. 7 + 0. 28 x - 0. 00055 x 2 70 r 2=0. 90 9. 4 =19% 60 50 40 30 20 40 60 80 N rate, kg/ha 100 120 140

Nitrogen Buffering Mechanisms 1. Increased Applied N results in increased plant N loss (NH 3) 2. Higher rates of applied N - increased volatilization losses 3. Higher rates of applied N - increased denitrification 4. Higher rates of applied N - increased organic C, - increased organic N 5. Increased applied N - increased grain protein 6. Increased applied N - increased forage N 7. Increased applied N - increased straw N

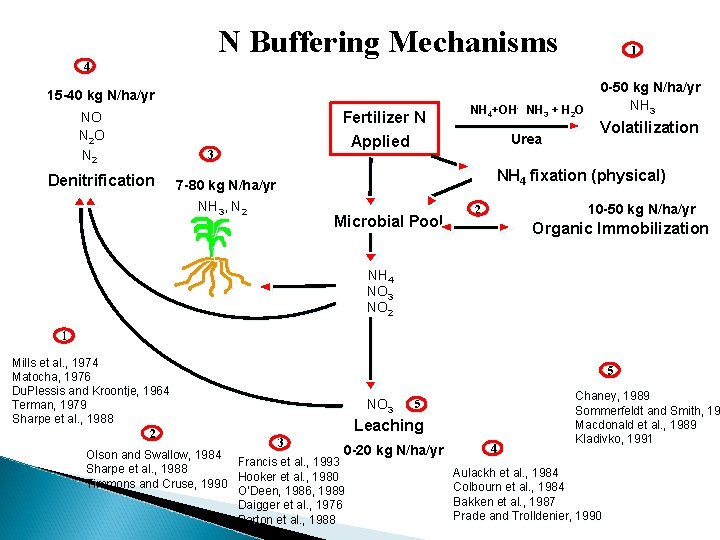
N Buffering Mechanisms 1 4 15 -40 kg N/ha/yr NO N 2 Denitrification 3 7 -80 kg N/ha/yr NH 3, N 2 Fertilizer N Applied NH 4+OH- NH 3 + H 2 O Urea 0 -50 kg N/ha/yr NH 3 Volatilization NH 4 fixation (physical) Microbial Pool 10 -50 kg N/ha/yr 2 Organic Immobilization NH 4 NO 3 NO 2 1 Mills et al. , 1974 Matocha, 1976 Du. Plessis and Kroontje, 1964 Terman, 1979 Sharpe et al. , 1988 2 5 NO 3 5 Leaching 3 0 -20 kg N/ha/yr Olson and Swallow, 1984 Francis et al. , 1993 Sharpe et al. , 1988 Hooker et al. , 1980 Timmons and Cruse, 1990 O’Deen, 1986, 1989 Daigger et al. , 1976 Parton et al. , 1988 4 Chaney, 1989 Sommerfeldt and Smith, 19 Macdonald et al. , 1989 Kladivko, 1991 Aulackh et al. , 1984 Colbourn et al. , 1984 Bakken et al. , 1987 Prade and Trolldenier, 1990

NITROGEN Cycle Links Industrial view of the Nitrogen Cycle Nutrient Overload: Unbalancing the Global Nitrogen Cycle Carbon Cycle

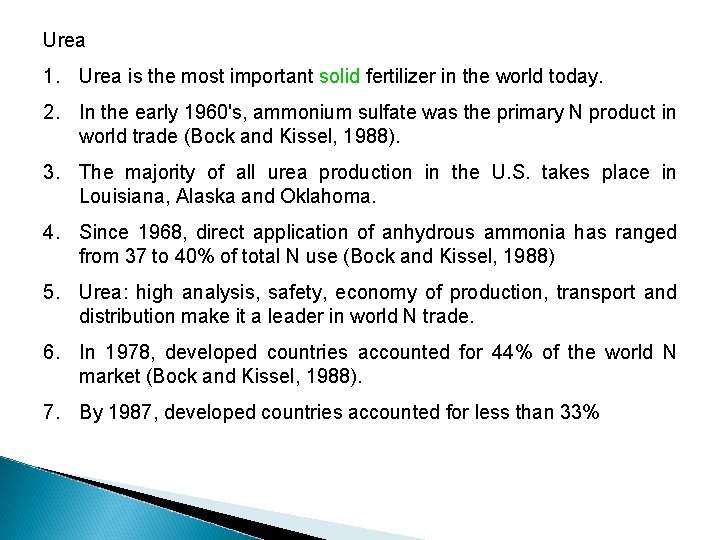
Urea 1. Urea is the most important solid fertilizer in the world today. 2. In the early 1960's, ammonium sulfate was the primary N product in world trade (Bock and Kissel, 1988). 3. The majority of all urea production in the U. S. takes place in Louisiana, Alaska and Oklahoma. 4. Since 1968, direct application of anhydrous ammonia has ranged from 37 to 40% of total N use (Bock and Kissel, 1988) 5. Urea: high analysis, safety, economy of production, transport and distribution make it a leader in world N trade. 6. In 1978, developed countries accounted for 44% of the world N market (Bock and Kissel, 1988). 7. By 1987, developed countries accounted for less than 33%

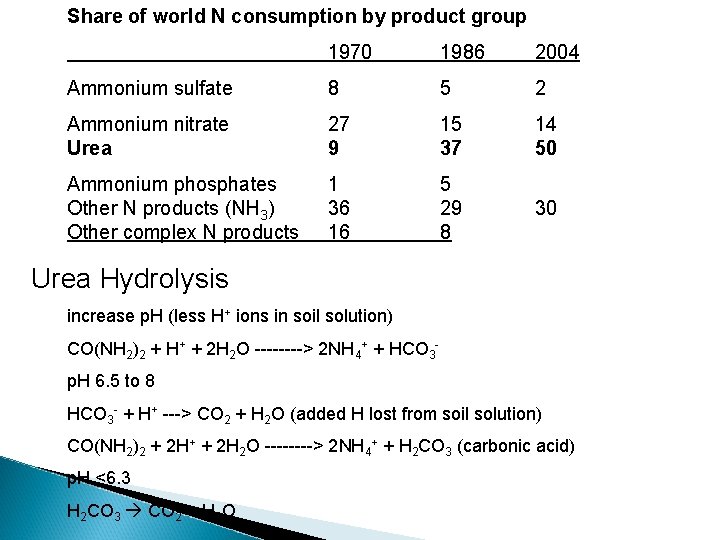
Share of world N consumption by product group 1970 1986 2004 Ammonium sulfate 8 5 2 Ammonium nitrate Urea 27 9 15 37 14 50 Ammonium phosphates Other N products (NH 3) Other complex N products 1 36 16 5 29 8 30 Urea Hydrolysis increase p. H (less H+ ions in soil solution) CO(NH 2)2 + H+ + 2 H 2 O ----> 2 NH 4+ + HCO 3 p. H 6. 5 to 8 HCO 3 - + H+ ---> CO 2 + H 2 O (added H lost from soil solution) CO(NH 2)2 + 2 H+ + 2 H 2 O ----> 2 NH 4+ + H 2 CO 3 (carbonic acid) p. H <6. 3 H 2 CO 3 CO 2 + H 2 O

During hydrolysis, soil p. H can increase to >7 because the reaction requires H+ from the soil system. (How many moles of H+ are consumed for each mole of urea hydrolyzed? ) 2 In alkaline soils less H+ is initially needed to drive urea hydrolysis on a soil already having low H+. In an alkaline soil, removing more H+(from a soil solution already low in H+), can increase p. H even higher NH 4+ + OH- ---> NH 4 OH ---->NH 3 + H 2 O p. H = -log[H+] Calculate p. H of 2. 0 x 10 -3 M solution of HCl is completely ionized so [H+] = 2. 0 x 10 -3 M p. H = -log(2. 0 x 10 -3) = 3 – log 2. 0 = 3 - 0. 30 = 2. 70
![p H p Ka log baseacid p Kw p ◦ p. H = p. Ka + log [(base)/(acid)] ◦ p. Kw = p.](https://slidetodoc.com/presentation_image_h/c957fac9948ed00881c28c716fdf1cd6/image-28.jpg)
◦ p. H = p. Ka + log [(base)/(acid)] ◦ p. Kw = p. H + p. OH ◦ 14. 00 = p. H + p. OH ◦ ◦ ◦ ◦ At a p. H of 9. 3 (p. Ka 9. 3) 50% NH 4 and 50% NH 3 p. H Base (NH 3) Acid (NH 4) 7. 3 1 99 8. 3 10 90 9. 3 50 50 10. 3 90 10 11. 3 99 1 Chemicals A and B react to form C and D A + B = C + D Equilibrium Constant (K) K = [C][D] / [A][B]

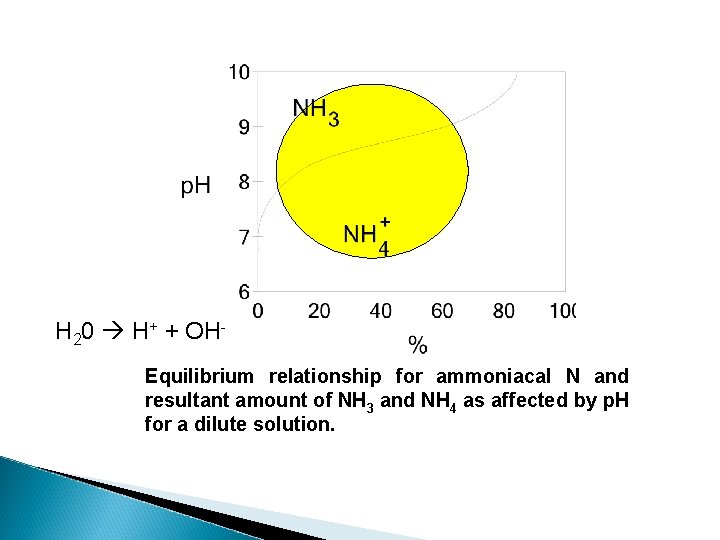
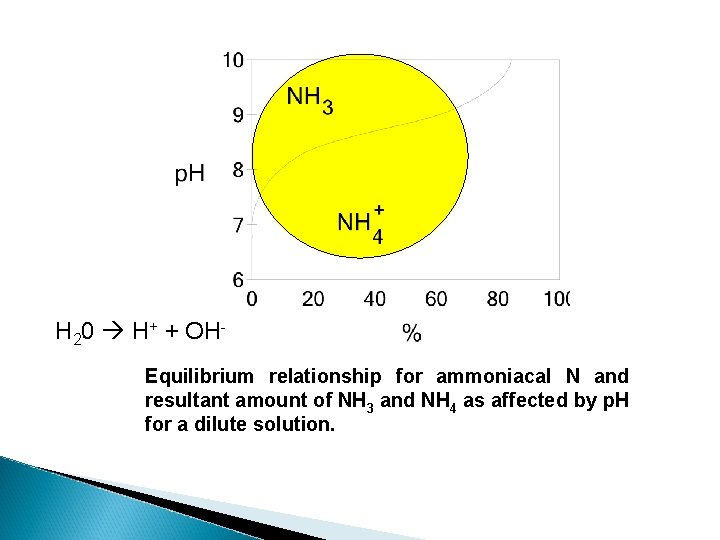
H 20 H+ + OHEquilibrium relationship for ammoniacal N and resultant amount of NH 3 and NH 4 as affected by p. H for a dilute solution.

As the p. H increases from urea hydrolysis, negative charges become available for NH 4+ adsorption because of the release of H+ (Koelliker and Kissel) Decrease NH 3 loss with increasing CEC (Fenn and Kissel, 1976) Assuming that p. H and CEC are positively correlated, what is happening? CEC p. H ** on soils where organic matter dominates the contribution to CEC then there should be a positive relationship of p. H and CEC. Relationship of p. H and BI (? ) none In acid soils, the exchange of NH 4+ is for H+ on the exchange complex (release of H here, resists change in p. H, e. g. going up) In alkaline soils with high CEC, NH 4 exchanges for Ca, precipitation of Ca. CO 3 (CO 3= from HCO 3 - above) and one H+ released which helps resist the increase in p. H However, p. H was already high,

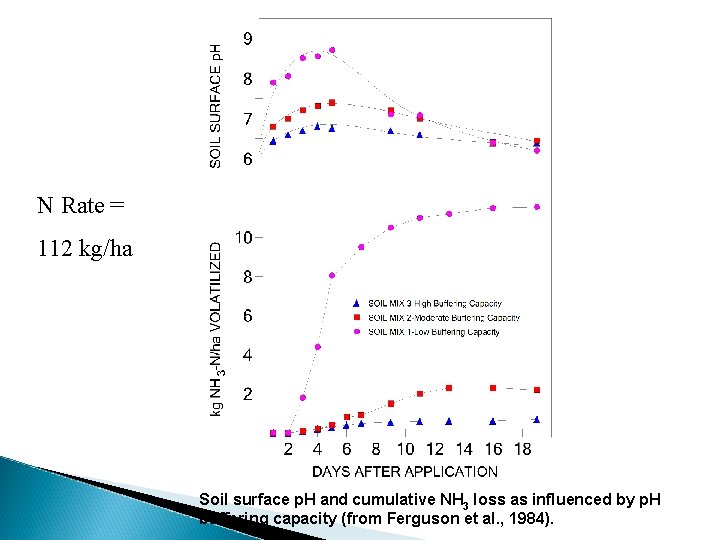
N Rate = 112 kg/ha Soil surface p. H and cumulative NH 3 loss as influenced by p. H buffering capacity (from Ferguson et al. , 1984).

Ernst and Massey (1960) found increased NH 3 volatilization when liming a silt loam soil. The effective CEC would have been increased by liming but the rise in soil p. H decreased the soils ability to supply H+ Rapid urea hydrolysis: greater potential for NH 3 loss. Why? Management: • dry soil surface • Incorporate • localized placement- slows urea hydrolysis

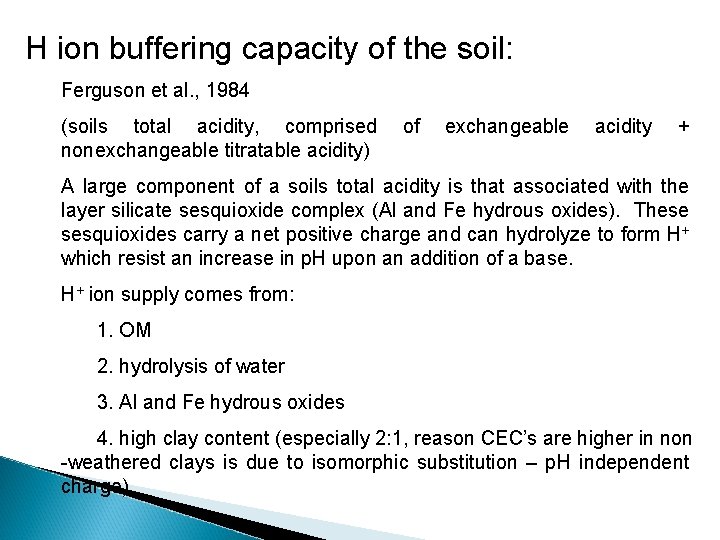
H ion buffering capacity of the soil: Ferguson et al. , 1984 (soils total acidity, comprised nonexchangeable titratable acidity) of exchangeable acidity + A large component of a soils total acidity is that associated with the layer silicate sesquioxide complex (Al and Fe hydrous oxides). These sesquioxides carry a net positive charge and can hydrolyze to form H+ which resist an increase in p. H upon an addition of a base. H+ ion supply comes from: 1. OM 2. hydrolysis of water 3. Al and Fe hydrous oxides 4. high clay content (especially 2: 1, reason CEC’s are higher in non -weathered clays is due to isomorphic substitution – p. H independent charge)

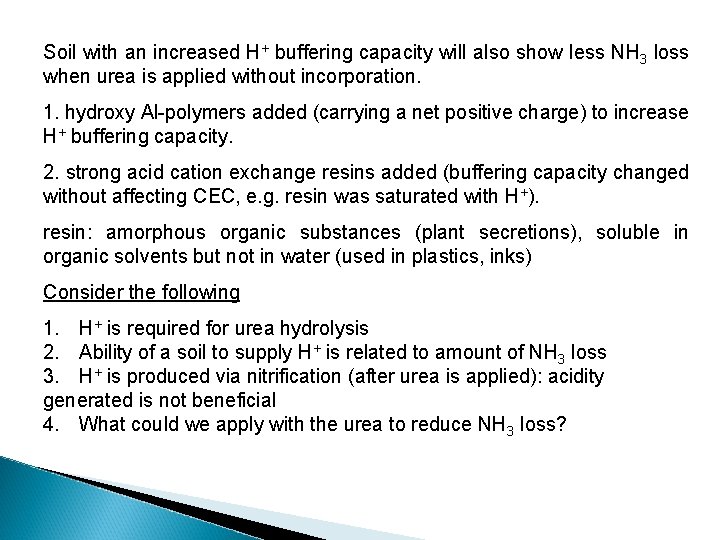
Soil with an increased H+ buffering capacity will also show less NH 3 loss when urea is applied without incorporation. 1. hydroxy Al-polymers added (carrying a net positive charge) to increase H+ buffering capacity. 2. strong acid cation exchange resins added (buffering capacity changed without affecting CEC, e. g. resin was saturated with H+). resin: amorphous organic substances (plant secretions), soluble in organic solvents but not in water (used in plastics, inks) Consider the following 1. H+ is required for urea hydrolysis 2. Ability of a soil to supply H+ is related to amount of NH 3 loss 3. H+ is produced via nitrification (after urea is applied): acidity generated is not beneficial 4. What could we apply with the urea to reduce NH 3 loss?

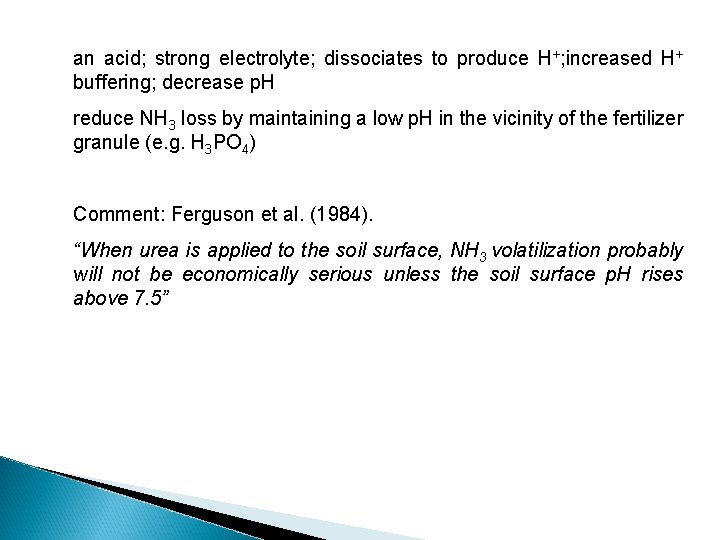
an acid; strong electrolyte; dissociates to produce H+; increased H+ buffering; decrease p. H reduce NH 3 loss by maintaining a low p. H in the vicinity of the fertilizer granule (e. g. H 3 PO 4) Comment: Ferguson et al. (1984). “When urea is applied to the soil surface, NH 3 volatilization probably will not be economically serious unless the soil surface p. H rises above 7. 5”

UREASE inhibitors “Agrotain” n-butyl thiophosphoric triamide http: //www. agrotain. com Nitrosomonas inhibitors “NSERVE” 2 -CHLORO-6 -(TRICHLOROMETHYL) PYRIDINE http: //jeq. scijournals. org/cgi/content/abstract/32/5/1764

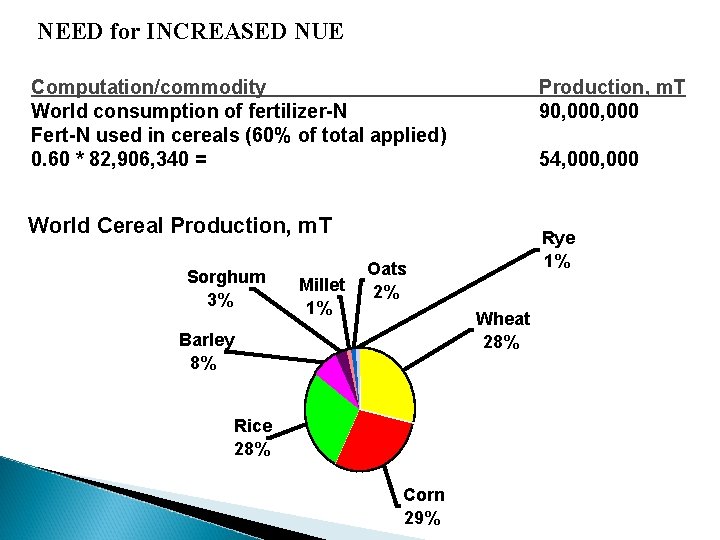
NEED for INCREASED NUE Computation/commodity World consumption of fertilizer-N Fert-N used in cereals (60% of total applied) 0. 60 * 82, 906, 340 = Production, m. T 90, 000 54, 000 World Cereal Production, m. T Sorghum 3% Millet 1% Rye 1% Oats 2% Wheat 28% Barley 8% Rice 28% Corn 29%

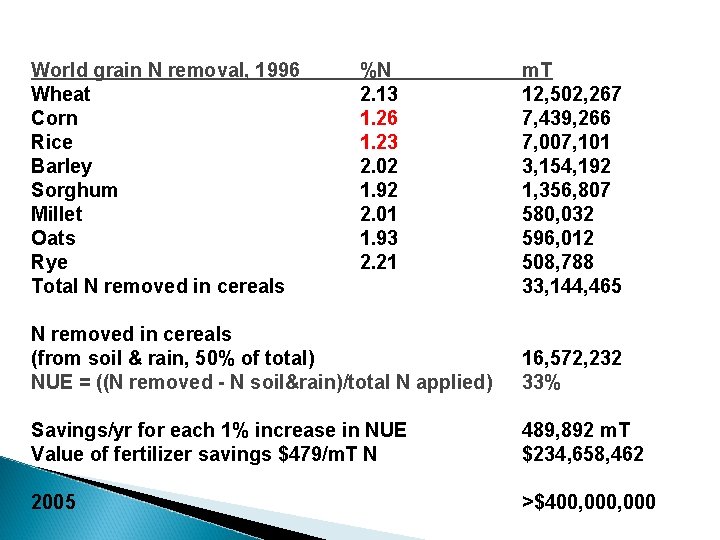
World grain N removal, 1996 Wheat Corn Rice Barley Sorghum Millet Oats Rye Total N removed in cereals %N 2. 13 1. 26 1. 23 2. 02 1. 92 2. 01 1. 93 2. 21 m. T 12, 502, 267 7, 439, 266 7, 007, 101 3, 154, 192 1, 356, 807 580, 032 596, 012 508, 788 33, 144, 465 N removed in cereals (from soil & rain, 50% of total) NUE = ((N removed - N soil&rain)/total N applied) 16, 572, 232 33% Savings/yr for each 1% increase in NUE Value of fertilizer savings $479/m. T N 489, 892 m. T $234, 658, 462 2005 >$400, 000

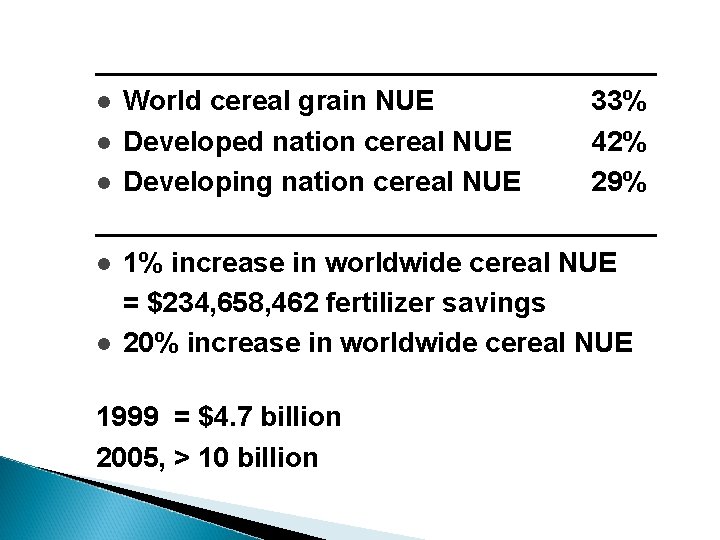
__________________ l World cereal grain NUE 33% l Developed nation cereal NUE 42% l Developing nation cereal NUE 29% __________________ l 1% increase in worldwide cereal NUE = $234, 658, 462 fertilizer savings l 20% increase in worldwide cereal NUE 1999 = $4. 7 billion 2005, > 10 billion

Flowchart for NUE http: //www. nue. okstate. edu/NUE_etc. htm

Role of NH 4 nutrition in Higher Yields (S. R. Olsen) • Glutamine-major product formed in roots absorbing NH 4 • NO 3 has to be transported to the leaves to be reduced • Wheat N uptake was increased 35% when supplying 25% of the N as NH 4 compared to all N as NO 3 (Wang and Below, 1992). • High-yielding corn genotypes were unable to absorb NO 3 during ear development, thus limiting yields otherwise increased by supplies of NH 4 (Pan et al. , 1984). • Assimilation of NO 3 requires the energy equivalent of 20 ATP/mole. NO 3, whereas NH 4 assimilation requires only 5 ATP/mole NH 4 (Salsac et al. , 1987). • This energy savings may lead to greater dry weight production for plants supplied solely with NH 4 (Huffman, 1989).

Bidwell (1979), Plant Physiology, 2 nd Ed. Metabolism associated with nitrate reduction photosynthesis carbohydrates respiration reducing power carbon skeletons NADH or NADPH NO 3 NO 2 nitrate reductase ferredoxin siroheme nitrite reductase NH 3 amino acids

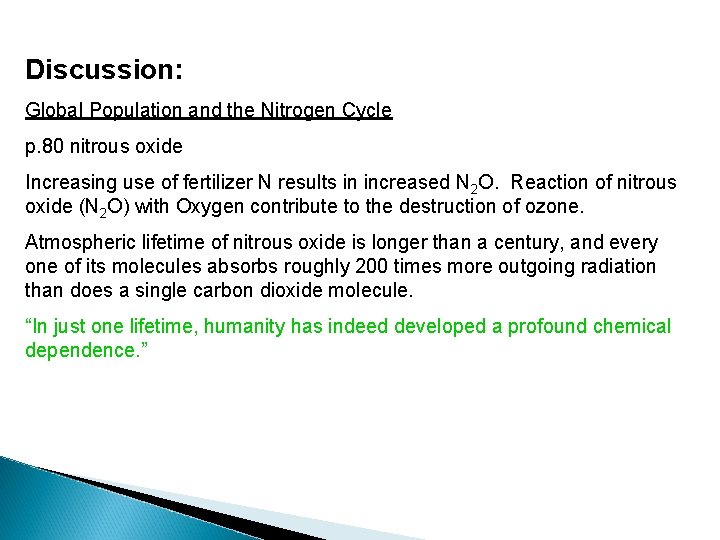
Discussion: Global Population and the Nitrogen Cycle p. 80 nitrous oxide Increasing use of fertilizer N results in increased N 2 O. Reaction of nitrous oxide (N 2 O) with Oxygen contribute to the destruction of ozone. Atmospheric lifetime of nitrous oxide is longer than a century, and every one of its molecules absorbs roughly 200 times more outgoing radiation than does a single carbon dioxide molecule. “In just one lifetime, humanity has indeed developed a profound chemical dependence. ”

FYI

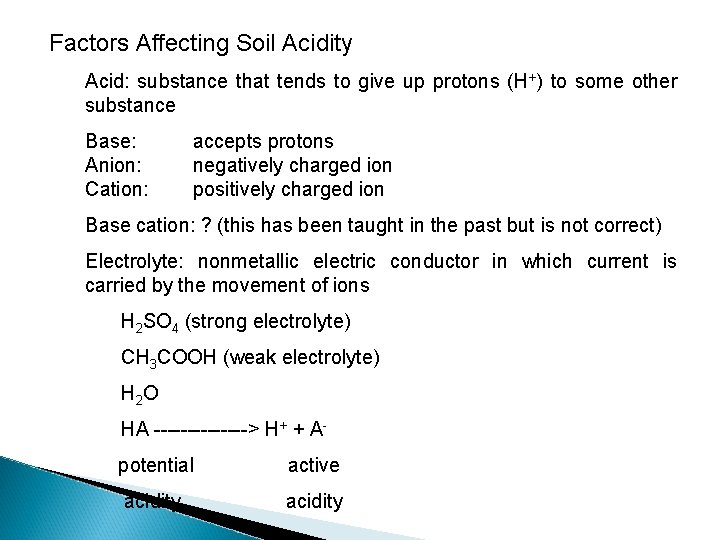
Factors Affecting Soil Acidity Acid: substance that tends to give up protons (H+) to some other substance Base: Anion: Cation: accepts protons negatively charged ion positively charged ion Base cation: ? (this has been taught in the past but is not correct) Electrolyte: nonmetallic electric conductor in which current is carried by the movement of ions H 2 SO 4 (strong electrolyte) CH 3 COOH (weak electrolyte) H 2 O HA -------> H+ + A potential active acidity

1. Nitrogen Fertilization A. ammoniacal sources of N 2. Decomposition of organic matter OM ------> R-NH 2 + CO 2 + H 2 O ----> H 2 CO 3 (carbonic acid) H 2 CO 3 ------> H+ + HCO 3 - (bicarbonate) humus contains reactive carboxylic, phenolic groups that behave as weak acids which dissociate and release H+

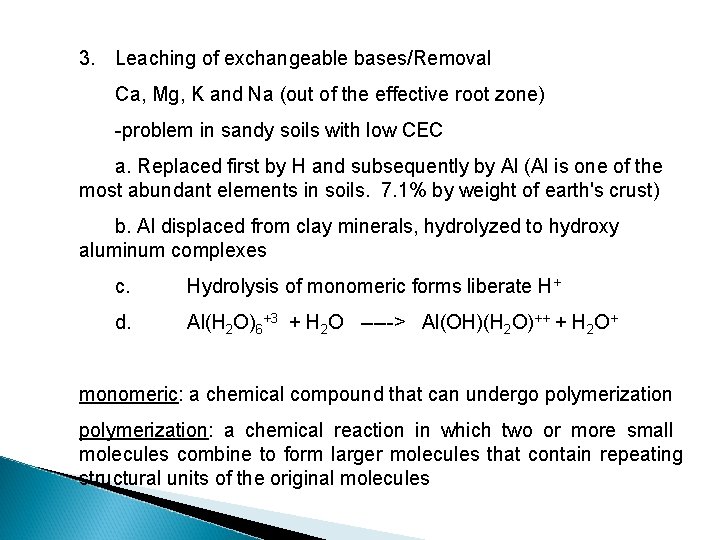
3. Leaching of exchangeable bases/Removal Ca, Mg, K and Na (out of the effective root zone) -problem in sandy soils with low CEC a. Replaced first by H and subsequently by Al (Al is one of the most abundant elements in soils. 7. 1% by weight of earth's crust) b. Al displaced from clay minerals, hydrolyzed to hydroxy aluminum complexes c. Hydrolysis of monomeric forms liberate H+ d. Al(H 2 O)6+3 + H 2 O -----> Al(OH)(H 2 O)++ + H 2 O+ monomeric: a chemical compound that can undergo polymerization: a chemical reaction in which two or more small molecules combine to form larger molecules that contain repeating structural units of the original molecules

4. Aluminosilicate clays Presence of exchangeable Al Al+3 + H 2 O -----> Al. OH= + H+ 5. Acid Rain

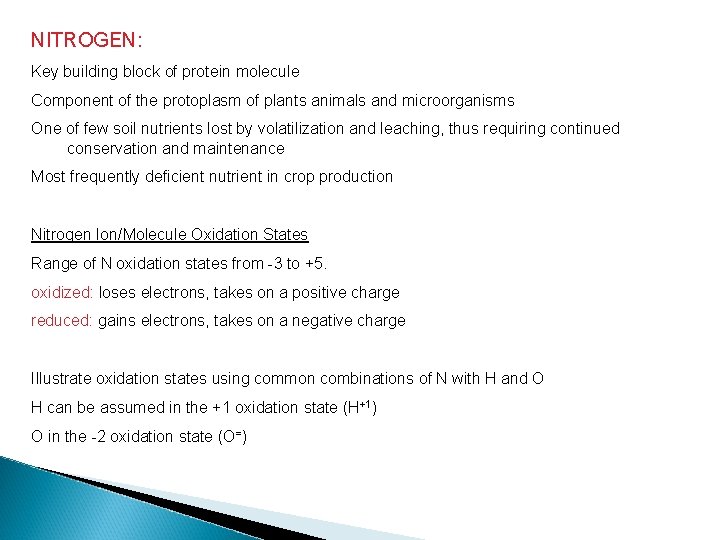
NITROGEN: Key building block of protein molecule Component of the protoplasm of plants animals and microorganisms One of few soil nutrients lost by volatilization and leaching, thus requiring continued conservation and maintenance Most frequently deficient nutrient in crop production Nitrogen Ion/Molecule Oxidation States Range of N oxidation states from -3 to +5. oxidized: loses electrons, takes on a positive charge reduced: gains electrons, takes on a negative charge Illustrate oxidation states using common combinations of N with H and O H can be assumed in the +1 oxidation state (H+1) O in the -2 oxidation state (O=)

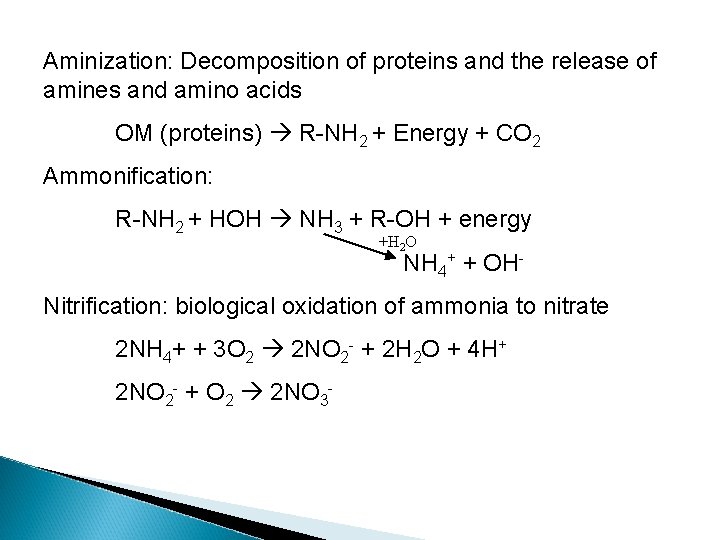
Aminization: Decomposition of proteins and the release of amines and amino acids OM (proteins) R-NH 2 + Energy + CO 2 Ammonification: R-NH 2 + HOH NH 3 + R-OH + energy +H 2 O NH 4+ + OH- Nitrification: biological oxidation of ammonia to nitrate 2 NH 4+ + 3 O 2 2 NO 2 - + 2 H 2 O + 4 H+ 2 NO 2 - + O 2 2 NO 3 -

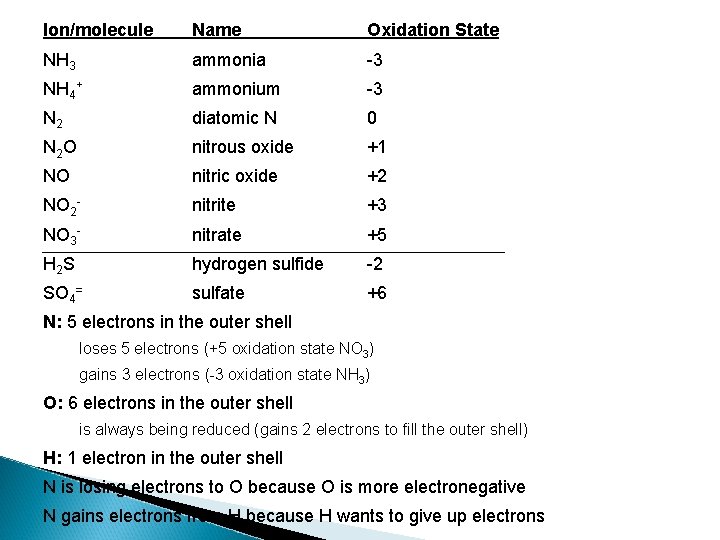
Ion/molecule Name Oxidation State NH 3 ammonia -3 NH 4+ ammonium -3 N 2 diatomic N 0 N 2 O nitrous oxide +1 NO nitric oxide +2 NO 2 - nitrite +3 NO 3 - nitrate +5 H 2 S hydrogen sulfide -2 SO 4= sulfate +6 N: 5 electrons in the outer shell loses 5 electrons (+5 oxidation state NO 3) gains 3 electrons (-3 oxidation state NH 3) O: 6 electrons in the outer shell is always being reduced (gains 2 electrons to fill the outer shell) H: 1 electron in the outer shell N is losing electrons to O because O is more electronegative N gains electrons from H because H wants to give up electrons

Hydrogen: Electron configuration in the ground state is 1 s 1 (the first electron shell has only one electron in it), as found in H 2 gas. s shell can hold only two electrons, atom is most stable by either gaining another electron or losing the existing one. Gaining an electron by sharing occurs in H 2, where each H atom gains an electron from the other resulting in a pair of electrons being shared. The electron configuration about the atom, where: represents a pair of electrons, and may be shown as H: H and the bond may be shown as H-H Hydrogen most commonly exists in ionic form and in combination with other elements where it has lost its single electron. Thus it is present as the H+ ion or brings a + charge to the molecule formed by combining with other elements.

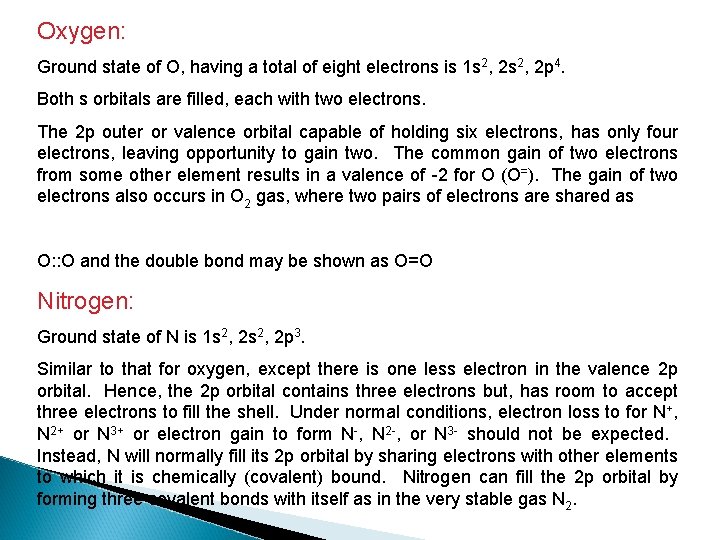
Oxygen: Ground state of O, having a total of eight electrons is 1 s 2, 2 p 4. Both s orbitals are filled, each with two electrons. The 2 p outer or valence orbital capable of holding six electrons, has only four electrons, leaving opportunity to gain two. The common gain of two electrons from some other element results in a valence of -2 for O (O=). The gain of two electrons also occurs in O 2 gas, where two pairs of electrons are shared as O: : O and the double bond may be shown as O=O Nitrogen: Ground state of N is 1 s 2, 2 p 3. Similar to that for oxygen, except there is one less electron in the valence 2 p orbital. Hence, the 2 p orbital contains three electrons but, has room to accept three electrons to fill the shell. Under normal conditions, electron loss to for N+, N 2+ or N 3+ or electron gain to form N-, N 2 -, or N 3 - should not be expected. Instead, N will normally fill its 2 p orbital by sharing electrons with other elements to which it is chemically (covalent) bound. Nitrogen can fill the 2 p orbital by forming three covalent bonds with itself as in the very stable gas N 2.

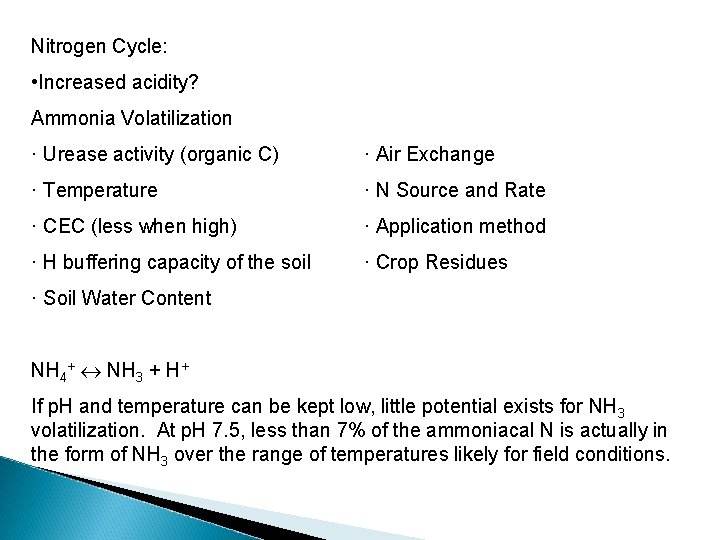
Nitrogen Cycle: • Increased acidity? Ammonia Volatilization · Urease activity (organic C) · Air Exchange · Temperature · N Source and Rate · CEC (less when high) · Application method · H buffering capacity of the soil · Crop Residues · Soil Water Content NH 4+ NH 3 + H+ If p. H and temperature can be kept low, little potential exists for NH 3 volatilization. At p. H 7. 5, less than 7% of the ammoniacal N is actually in the form of NH 3 over the range of temperatures likely for field conditions.

H 20 H+ + OHEquilibrium relationship for ammoniacal N and resultant amount of NH 3 and NH 4 as affected by p. H for a dilute solution.

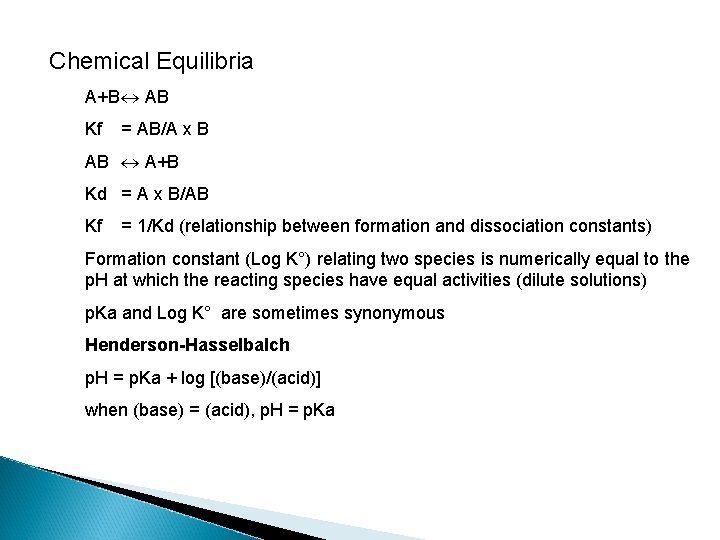
Chemical Equilibria A+B AB Kf = AB/A x B AB A+B Kd = A x B/AB Kf = 1/Kd (relationship between formation and dissociation constants) Formation constant (Log K°) relating two species is numerically equal to the p. H at which the reacting species have equal activities (dilute solutions) p. Ka and Log K° are sometimes synonymous Henderson-Hasselbalch p. H = p. Ka + log [(base)/(acid)] when (base) = (acid), p. H = p. Ka

Acidification from N Fertilizers (R. L. Westerman) 1. Assume that the absorbing complex of the soil can be represented by Ca. X 2. Ca represents various exchangeable bases with which the insoluble anions X are combined in an exchangeable form and that X can only combine with one Ca 3. H 2 X refers to dibasic acid (e. g. , H 2 SO 4) (NH 4)2 SO 4 -----> NH 4+ to the exchange complex, SO 4= combines with the base on the exchange complex replaced by NH 4+ Volatilization losses of N as NH 3 preclude the development of H+ ions produced via nitrification and would theoretically reduce the total potential development of acidity. Losses of N via denitrification leave an alkaline residue (OH-)

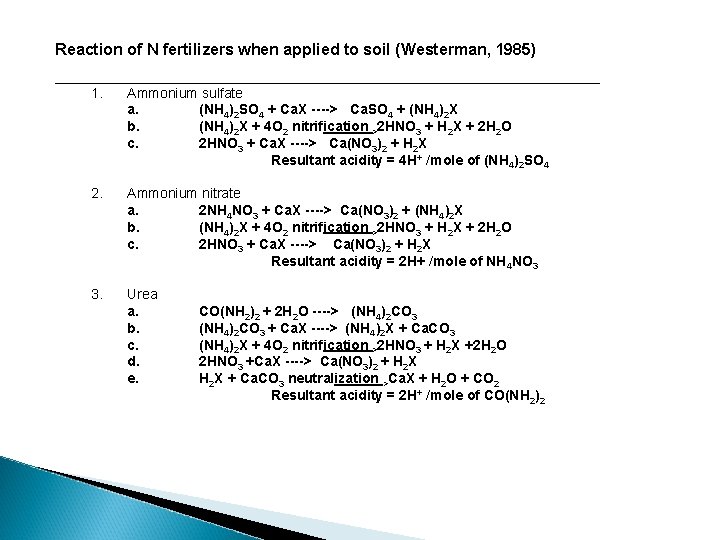
Reaction of N fertilizers when applied to soil (Westerman, 1985) ___________________________________ 1. Ammonium sulfate a. (NH 4)2 SO 4 + Ca. X ----> Ca. SO 4 + (NH 4)2 X b. (NH 4)2 X + 4 O 2 nitrification >2 HNO 3 + H 2 X + 2 H 2 O c. 2 HNO 3 + Ca. X ----> Ca(NO 3)2 + H 2 X Resultant acidity = 4 H+ /mole of (NH 4)2 SO 4 2. Ammonium nitrate a. 2 NH 4 NO 3 + Ca. X ----> Ca(NO 3)2 + (NH 4)2 X b. (NH 4)2 X + 4 O 2 nitrification >2 HNO 3 + H 2 X + 2 H 2 O c. 2 HNO 3 + Ca. X ----> Ca(NO 3)2 + H 2 X Resultant acidity = 2 H+ /mole of NH 4 NO 3 3. Urea a. b. c. d. e. CO(NH 2)2 + 2 H 2 O ----> (NH 4)2 CO 3 + Ca. X ----> (NH 4)2 X + Ca. CO 3 (NH 4)2 X + 4 O 2 nitrification >2 HNO 3 + H 2 X +2 H 2 O 2 HNO 3 +Ca. X ----> Ca(NO 3)2 + H 2 X + Ca. CO 3 neutralization >Ca. X + H 2 O + CO 2 Resultant acidity = 2 H+ /mole of CO(NH 2)2

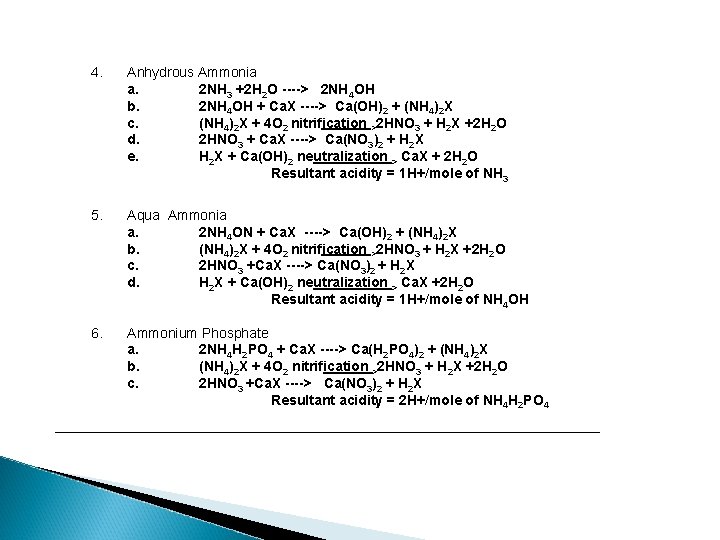
4. Anhydrous Ammonia a. 2 NH 3 +2 H 2 O ----> 2 NH 4 OH b. 2 NH 4 OH + Ca. X ----> Ca(OH)2 + (NH 4)2 X c. (NH 4)2 X + 4 O 2 nitrification >2 HNO 3 + H 2 X +2 H 2 O d. 2 HNO 3 + Ca. X ----> Ca(NO 3)2 + H 2 X e. H 2 X + Ca(OH)2 neutralization > Ca. X + 2 H 2 O Resultant acidity = 1 H+/mole of NH 3 5. Aqua Ammonia a. 2 NH 4 ON + Ca. X ----> Ca(OH)2 + (NH 4)2 X b. (NH 4)2 X + 4 O 2 nitrification >2 HNO 3 + H 2 X +2 H 2 O c. 2 HNO 3 +Ca. X ----> Ca(NO 3)2 + H 2 X d. H 2 X + Ca(OH)2 neutralization > Ca. X +2 H 2 O Resultant acidity = 1 H+/mole of NH 4 OH 6. Ammonium Phosphate a. 2 NH 4 H 2 PO 4 + Ca. X ----> Ca(H 2 PO 4)2 + (NH 4)2 X b. (NH 4)2 X + 4 O 2 nitrification >2 HNO 3 + H 2 X +2 H 2 O c. 2 HNO 3 +Ca. X ----> Ca(NO 3)2 + H 2 X Resultant acidity = 2 H+/mole of NH 4 H 2 PO 4 ___________________________________
 Carbon and nitrogen cycling in soil:
Carbon and nitrogen cycling in soil: Venkatraman ramakrishnan medium
Venkatraman ramakrishnan medium Nutrient cycle of a tropical rainforest
Nutrient cycle of a tropical rainforest Nutrient cycle in the serengeti
Nutrient cycle in the serengeti Gersmehl model deciduous woodland
Gersmehl model deciduous woodland Who coined ecosystem
Who coined ecosystem Mineral cycle diagram
Mineral cycle diagram From where nitrogen comes in atmosphere
From where nitrogen comes in atmosphere Living soil vs dead soil
Living soil vs dead soil Four major spheres of the earth
Four major spheres of the earth Nitrogen cycle vocabulary
Nitrogen cycle vocabulary Nitrogen cycle
Nitrogen cycle Nitrogen cycle interactive
Nitrogen cycle interactive Summarize the water cycle
Summarize the water cycle Nitrogen cycle pearson education
Nitrogen cycle pearson education Nitrogen cycle diagram leaving cert
Nitrogen cycle diagram leaving cert Nature cycle
Nature cycle Summary of the nitrogen cycle
Summary of the nitrogen cycle Nitrogen cycyle
Nitrogen cycyle Ammonification
Ammonification Importance of nitrogen cycle
Importance of nitrogen cycle Conclusion of nitrogen cycle
Conclusion of nitrogen cycle Nitrogen cycle comic strip
Nitrogen cycle comic strip Nitrogen cycle cow
Nitrogen cycle cow Cycle alliteration
Cycle alliteration Nitrogen cycle
Nitrogen cycle Ap environmental science biogeochemical cycles
Ap environmental science biogeochemical cycles How does carbon enter the atmosphere
How does carbon enter the atmosphere What is nitrogen
What is nitrogen Vernon scannell nettles
Vernon scannell nettles Redfield ratio
Redfield ratio Nitrogen in macromolecules
Nitrogen in macromolecules Nitrogen cycle diagram simple
Nitrogen cycle diagram simple A bird stalks kills then eats
A bird stalks kills then eats Noaa hysplit
Noaa hysplit Matter cycle
Matter cycle Section 3 cycling of matter answer key
Section 3 cycling of matter answer key Quantitative research about cycling
Quantitative research about cycling Tpn cycling
Tpn cycling Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem Cycling event sponsorship proposal
Cycling event sponsorship proposal Road cycling 101
Road cycling 101 Buying and disposing consumer behavior
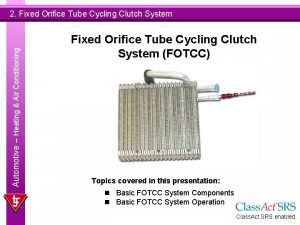
Buying and disposing consumer behavior Cycling clutch orifice tube
Cycling clutch orifice tube Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem The cycling of materials
The cycling of materials Principles of ecology organisms and their relationships
Principles of ecology organisms and their relationships Matter cycling in ecosystems
Matter cycling in ecosystems Chapter 2 section 1 organisms and their relationships
Chapter 2 section 1 organisms and their relationships Principles of ecology chapter 2 section 1 answer key
Principles of ecology chapter 2 section 1 answer key Castleknock cycling club
Castleknock cycling club Cycling of matter definition biology
Cycling of matter definition biology Hasselt tourism
Hasselt tourism Guide to cycling
Guide to cycling Cycling to work
Cycling to work Chemical cycling in an ecosystem
Chemical cycling in an ecosystem Energy flow in cellular respiration
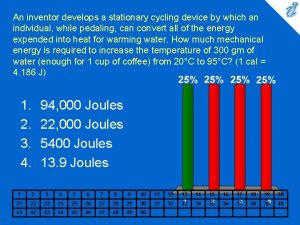
Energy flow in cellular respiration An inventor develops a stationary cycling device
An inventor develops a stationary cycling device Cycling seniors
Cycling seniors Same pattern words
Same pattern words