6 Exchange SOIL 5813 SoilPlant Nutrient Cycling and











- Slides: 21

6. Exchange SOIL 5813 Soil-Plant Nutrient Cycling and Environmental Quality Department of Plant and Soil Sciences Oklahoma State University Stillwater, OK 74078 email: wrr@mail. pss. okstate. edu Tel: (405) 744 -6414

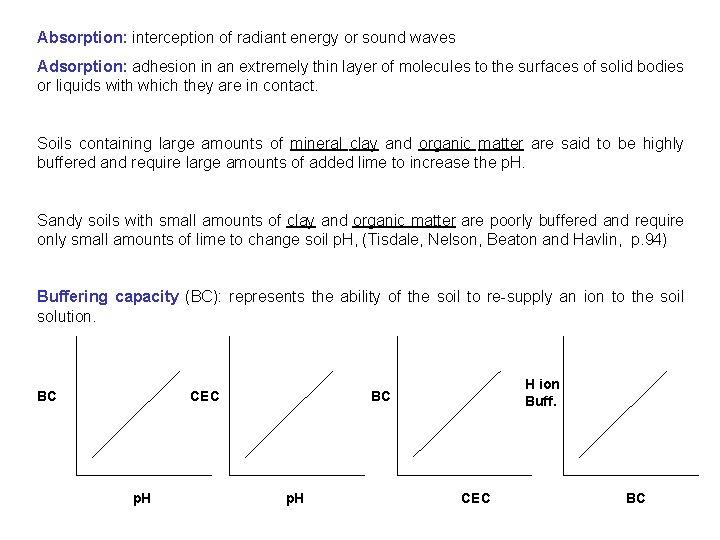
Absorption: interception of radiant energy or sound waves Adsorption: adhesion in an extremely thin layer of molecules to the surfaces of solid bodies or liquids with which they are in contact. Soils containing large amounts of mineral clay and organic matter are said to be highly buffered and require large amounts of added lime to increase the p. H. Sandy soils with small amounts of clay and organic matter are poorly buffered and require only small amounts of lime to change soil p. H, (Tisdale, Nelson, Beaton and Havlin, p. 94) Buffering capacity (BC): represents the ability of the soil to re-supply an ion to the soil solution. BC CEC p. H H ion Buff. BC p. H CEC BC

p. H independent charge (permanent) Isomorphic substitution: substitution of one element for another in ionic crystals without changing the structure of the crystal a. Substitution of Al+++ for Si++++ in tetrahedral b. Mg++, Fe+++ for Al+++ in octahedral c. Leaves a net negative charge (permanent) d. p. H dependent charge: positive charge developed at low p. H and excess negative charge formed at high p. H e. Gain or loss of H+ from functional groups on the surface of soil solids. f. Hydroxy (-OH) g. Carboxyl (-COOH) h. Phenolic (-C 6 H 4 OH)

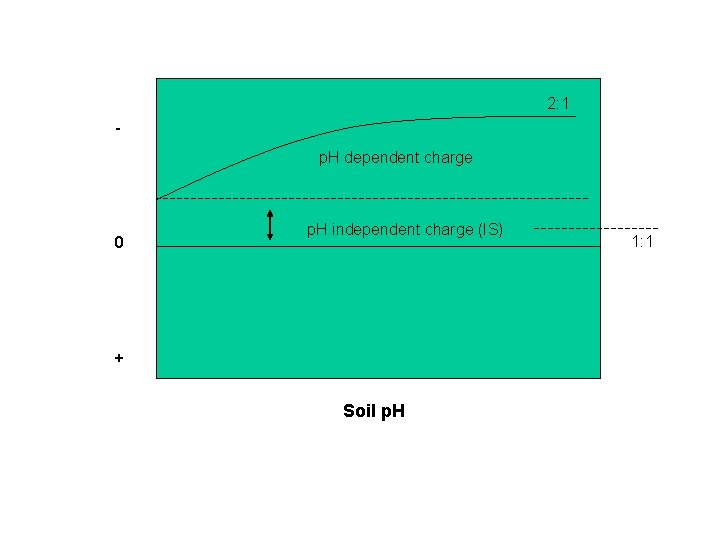
p. H dependent charge (cont. ) Only 5 -10% of the negative charge on 2: 1 layer silicates is p. H dependent whereas 50% or more of the charge developed on 1: 1 minerals can be p. H dependent. 1. Kaolinite: 1: 1 At high p. H, these H+ are weakly held and may be exchanged. At low p. H, H+ are held very tightly and are not exchanged. Deprotonation or dissociation of H+ from OH- groups at the broken edges of clay particles is the prime source of negative charge in the 1: 1 clay minerals. High p. H values favor this deprotonation of exposed hydroxyl groups. This creates some confusion since high p. H is seldom associated with weathered soils. Fifty percent or more of the charge developed on 1: 1 clay minerals can be p. H dependent. In a weathered soil, hydrous oxides (Fe and Al oxides) are a more important source of p. H dependent charge. Si (as silicic acid H 4 Si. O 4) is weathered and leached. 2: 1’s become 1: 1’s become Fe-Al oxides Where does the negative charge come from in an acid soil? 2. Organic Matter Most soils have a net negative charge due to negative charges on layer silicates and organic matter, however, some highly weathered soils dominated by allophane and hydrous oxides may actually have a net positive charge at low p. H.

Si. O- permanent charge (will form Si. OH but at p. H 2. 0) p. H dependent charges Al-OH p. H = 6 – 7. 5 (pure surface) Al-O- p. H goes up (negative charge) Al. OH 2+ p. H goes down (positive charge) Same for Fe

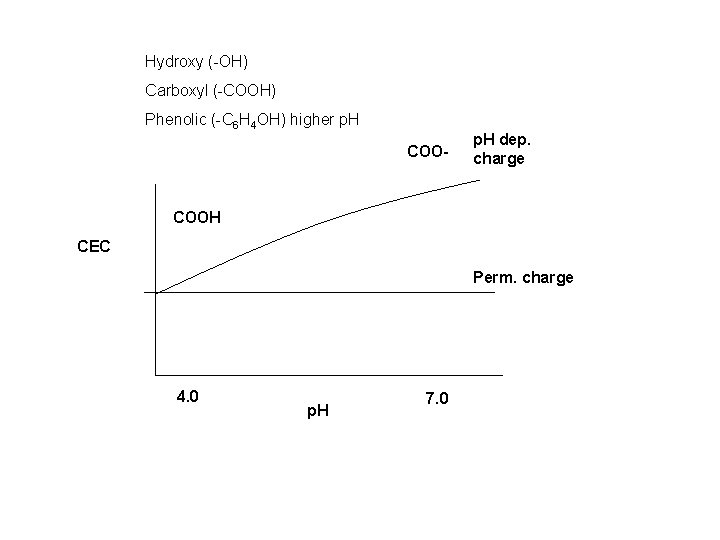
Hydroxy (-OH) Carboxyl (-COOH) Phenolic (-C 6 H 4 OH) higher p. H COO- p. H dep. charge COOH CEC Perm. charge 4. 0 p. H 7. 0

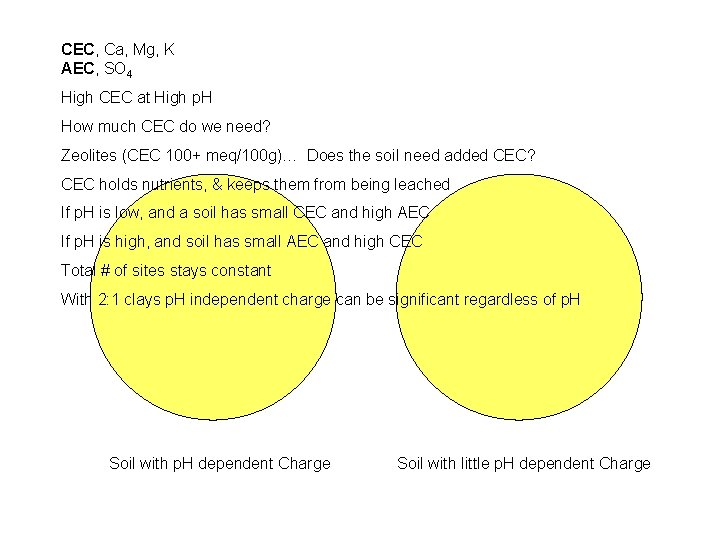
CEC, Ca, Mg, K AEC, SO 4 High CEC at High p. H How much CEC do we need? Zeolites (CEC 100+ meq/100 g)… Does the soil need added CEC? CEC holds nutrients, & keeps them from being leached If p. H is low, and a soil has small CEC and high AEC If p. H is high, and soil has small AEC and high CEC Total # of sites stays constant With 2: 1 clays p. H independent charge can be significant regardless of p. H Soil with p. H dependent Charge Soil with little p. H dependent Charge

2: 1 p. H dependent charge 0 p. H independent charge (IS) + Soil p. H 1: 1

Should not use a buffered solution (fixed p. H) for CEC. If a 1 N NH 4 OAc ( p. H>7. 0 ) solution were used to displace cations on the exchange complex of a soil with a p. H of 5. 0, CEC would be overestimated as p. H dependent charge sites would be included (specifically organic matter) that would not have been present at the soils natural p. H. Calcareous soil, REVERSE Ions must exist in soils as solid compounds or adsorbed to cation/anion exchange sites. Can be described by the ratio of the concentrations of absorbed (D Q) and solution (D I) ions; BC = D Q/D I The BC in soil increases with increasing CEC, organic matter and other solid constituents in the soil. For most minerals the strength of cation adsorption or lyotropic series is: Al+++>Ca++>Mg++>K+=NH 4+>Na+ ions with a higher valence are held more tightly than monovalent cations (exception, H+) Al+++>H+>Ca++>Mg++>K+=NH 4+>Na+

Replaceability of an ion decreases as its dehydrated radius increases. Cations are attracted toward, and anions are repelled from, negatively charged soil colloids. These interactions follow Coulomb's law where; F=qq'/Dr 2 F is the force of attraction or repulsion q and q 1 are the electrical charges (esu, equal to 2. 09 x 109 individual electronic charges) r is the distance of charge separation (cm) D is the dielectric constant (=78 for water at 25°C) Strength of ion retention or repulsion increases with increasing ion charge, with increasing colloid charge and with decreasing distance between the colloid surface and either the source of charge or the soluble ion. Interaction between ions increases with concentration and with the square of the ion charge. The parameter embracing the concentration and charge effects is the ionic strength (I) of the solution. I = ½ sum Mi Zi 2 where M is the molarity, Z is the charge of each ion i. Ionic strength measures the effective ion concentration by taking into account the pronounced effect of ion charge on solution properties. A solution has only one ionic strength but each of its constituent ions may have a different activity coefficient.

Exchangeable cations: Ca++ Mg++ K+ and Na+ Exchangeable acidity: 1. H ions obtained from the hydrolysis of exchangeable, trivalent Al 2. Hydrolysis of partially hydrolyzed and non-exchangeable Al 3. Weakly acidic groups, mostly on organic matter 4. Exchangeable H In the early days of soil science there was no agreement on the p. H of the soil at which exchangeable acidity was to be determined. Bradfield, 1923 noted that the usual substance used to increase the p. H of acid soils is Ca. CO 3 and that the maximum p. H obtainable with Ca. CO 3 is p. H 8. 3. Therefore base saturation is defined as the quantity of base adsorbed by a soil in the presence of Ca. CO 3 equilibrated with air having a CO 2 content of 0. 03% (Thomas, 1982).
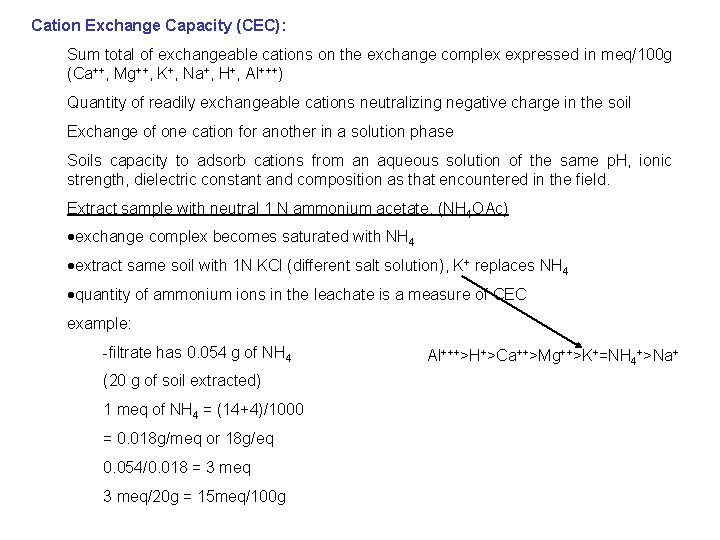
Cation Exchange Capacity (CEC): Sum total of exchangeable cations on the exchange complex expressed in meq/100 g (Ca++, Mg++, K+, Na+, H+, Al+++) Quantity of readily exchangeable cations neutralizing negative charge in the soil Exchange of one cation for another in a solution phase Soils capacity to adsorb cations from an aqueous solution of the same p. H, ionic strength, dielectric constant and composition as that encountered in the field. Extract sample with neutral 1 N ammonium acetate. (NH 4 OAc) ·exchange complex becomes saturated with NH 4 ·extract same soil with 1 N KCl (different salt solution), K+ replaces NH 4 ·quantity of ammonium ions in the leachate is a measure of CEC example: -filtrate has 0. 054 g of NH 4 (20 g of soil extracted) 1 meq of NH 4 = (14+4)/1000 = 0. 018 g/meq or 18 g/eq 0. 054/0. 018 = 3 meq/20 g = 15 meq/100 g Al+++>H+>Ca++>Mg++>K+=NH 4+>Na+

increase clay, increase CEC increase OM, increase CEC increase 2: 1, increase CEC 1: 1 clays: 1 -10 meq/100 g 2: 1 clays: 80 -150 meq/100 g

Effective CEC Extraction with an unbuffered salt which would give a measure of the CEC at the soils normal p. H. Use of neutral N ammonium acetate (7. 0) will result in a high CEC on acid soils because of the adsorption of NH 4 to the p. H dependent charge sites. Why? 1. At high p. H, H+ are weakly held and may be exchanged; p. H dependent charge 2. Deprotonation (dissociation of H from OH groups at the broken edges of clay particles which is the prime source of negative charge in 1: 1 clay minerals) occurs only at high p. H (7. 0 and up) Kamprath: unbuffered salt solution, 1. 0 N KCl will extract only the cations held at active exchange sites at the particular p. H of the soil. The exchangeable acidity is due to Al and H.

CEC Methods 1. Polemio & Rhoades (1977) arid soils containing carbonates, gypsum and zeolites. Saturation of exchange sites with Na (p. H 8. 2) 0. 4 N Na. OAc + 0. 1 N Na. Cl Extraction with 0. 5 N Mg. NO 3 Na determined (soluble Na from saturation step deducted from total Na to obtain exchangeable Na) Method will determine CEC as a result of permanent charge but not for variable charged soils (p. H) ** adding NH 4 OAc to a calcareous soil would result in NH 3 volatilization ** at p. H 7, using NH 4 OAc, NH 4 will not displace all of the Ca in a calcareous soil (underestimate CEC) ** at p. H 8. 2 (or higher), Ca. CO 3 will not dissolve anymore 2. Gillman (1979) acid soils (Ba has a higher charge density than does NH 4 (more charge per volume)) Saturation of exchange sites with Ba. Cl 2 (solution of a concentration approximately equivalent in ionic strength to the soil solution) Extraction with Mg. SO 4 to replace Ba with Mg (Mg. SO 4 concentration is adjusted to achieve an ionic strength comparable with that of the soil solution) Ba determined The use of unbuffered solutions throughout ensures that natural soil p. H is not significantly altered. SCS (has largely determined benchmark methods simply due to volume of samples over the years) NH 4 OAc at p. H 7. 0

CEC Problems Presence of Ca. CO 3 and/or Ca. SO 4 (dissolution) and the presence of salt in arid type soils. Dissolution of Ca. CO 3 and/or Ca. SO 4 will cause Ca to exchange for Mg, K and Na instead of NH 4 replacing all of these. When 1 N KCl is then added to displace the NH 4 (from NH 4 OAc) less NH 4 is detected in the filtrate than what should have been present. Variable charge soils (high content of more difficulty exchangeable aluminum-hydroxy "cations"). Exchangeable Al and its hydroxy forms are not readily exchanged with monovalent cation saturation solutions. This error results in an underestimation of CEC. The underlying factor which has caused various researchers to develop alternative methods for determining CEC was how to deal with p. H dependent charges (p. H of the saturating solution and replacement solution). This is important considering the p. H is a logarithmic function of H+ where 10 times as much H occurs in solution at p. H 5 as p. H 6. Schollenberger (1936) chose NH 4 because NH 4 levels were low in soils Ba was not used because the emission line for Ba is very close to K (766. 5 nm) Flame photometers were used from 1950 to 1970 Atomic absorption did not have the interference (could now use Ba to extract Al)

Base Saturation Why is it important to know Base Saturation? Should probably use exchangeable acidity (K is supplied via the CEC, so should we be more interested in exchangeable K) If BS is high (>70%), don’t worry about Ca, Mg and K If BS is low (30%), worry about Al BASE SATURATION USED in MORPHOLOGY BS of 35% or more at a depth of 0. 75 to 1. 25 m (Alfisol) BS of < 35% 0. 75 to 1. 25 m (Ultisol)

BASE SATURATION: Reflects the extent of leaching and weathering of the soil? Could have high BS and high Na…. What does this mean? Drainage? It is the percentage of total CEC occupied by cations, Ca++, Mg++, Na+ and K+, where each is determined separately from the NH 4 OAc extract (Atomic Absorption - interception of radiant energy) Ca 0. 03 g Mg 0. 008 g Na 0. 021 g K 0. 014 g Ca = 0. 03/0. 02 = 1. 5 Mg = 0. 008/0. 012 = 0. 66 Na = 0. 021/0. 023 = 0. 91 K = 0. 014/0. 039 = 0. 36 =3. 43 meq/20 g =17. 15 meq/100 g CEC = 20 meq/100 g BS = 17. 15/20 = 85. 85% BS = CEC - (H+ + Al+++) / CEC * remember this is exchangeable H+ and Al+++

Anion Exchange (Kamprath) Adsorption of anions to + charged sites in hydrous oxide minerals where the hydrous oxides are amphoteric (have - and + charge depending on p. H and therefore have AEC and CEC). Order of adsorption strength H 2 PO 4 - > SO 4= >NO 3 - = Clp. H < 7. 0 More in weathered soils (1: 1) containing hydrous oxides of Fe and Al (exposed OH groups on the edges of clay minerals) Soils which have p. H dependent charges. Anion exchange of 43 meq/100 g at an acidic equilibrium p. H of 4. 7. Can a soil have a net positive charge? (highly acid < 5. 0, Fe. O, Oxisols) Is H 2 PO 4 - adsorption on soils anion exchange? only physically adsorbed initially but soon precipitate as Ca-P in alkaline soils and Fe or Al-P in acid soils. Can P applications induce S deficiencies in acid soils? Acid soil: S levels low --> P exchange for S on exchange complex (anion exchange) and SO 4= can be leached. 90% of all water soluble bases will be leached as sulfate (Pearson et al, 1962)

Kamprath et al. (1956) Increased P concentration in solution reduced the amounts of SO 4= adsorbed by the soil. Amount of sulfate adsorbed decreased as the p. H of the soil suspension increased (4 to 6). Aylmore et al. (1967) Sulfate adsorption on clays possessing positive edge charges + oxides of Fe and Al (highly resistant to leaching and less available for plant growth) Sulfate adsorbed on kaolinite clay is weakly held and easily released Fox et al. (1964) Ca(H 2 PO 4)2 best extracting solution for S AEC negatively correlated with Base Saturation

Discussion: p. H and BS are positively correlated Why would p. H and BS be positively correlated if p. H and CEC were not? All are + positively correlated (acid soils would be the exception) If CEC, Base Saturation, Buffering Capacity, Hydrogen ion buffering capacity are all positively correlated, why don’t we just use one procedure for all of them? Are CEC and AEC negatively correlated?
 Transport media for mycobacterium tuberculosis
Transport media for mycobacterium tuberculosis Carbon and nitrogen cycling in soil:
Carbon and nitrogen cycling in soil: Living soil vs dead soil
Living soil vs dead soil What are the four spheres of the earth
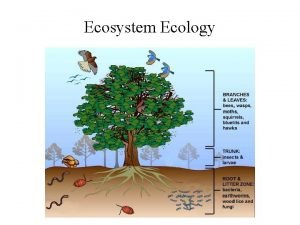
What are the four spheres of the earth Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem Mary likes hiking swimming and cycling
Mary likes hiking swimming and cycling Choose the sentence that demonstrates parallel structure
Choose the sentence that demonstrates parallel structure Mary likes hiking swimming and to ride a bicycle
Mary likes hiking swimming and to ride a bicycle Addison currency exchange
Addison currency exchange Voluntary exchange activity the pearl exchange
Voluntary exchange activity the pearl exchange Gas exchange key events in gas exchange
Gas exchange key events in gas exchange A bird stalks kills then eats
A bird stalks kills then eats Cycling swanier
Cycling swanier Cycling of matter in an ecosystem
Cycling of matter in an ecosystem Section 3 cycling of matter answer key
Section 3 cycling of matter answer key Quantitative research about cycling
Quantitative research about cycling Tpn tapering guidelines
Tpn tapering guidelines Cycling event sponsorship proposal
Cycling event sponsorship proposal Road cycling 101
Road cycling 101 Buying and disposing consumer behavior
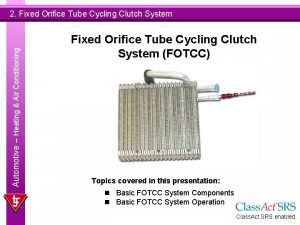
Buying and disposing consumer behavior Cycling clutch orifice tube
Cycling clutch orifice tube