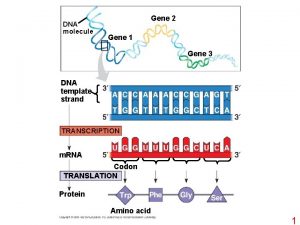
3 12 Gene Knockouts and Transgenics transgenics Organisms









- Slides: 51

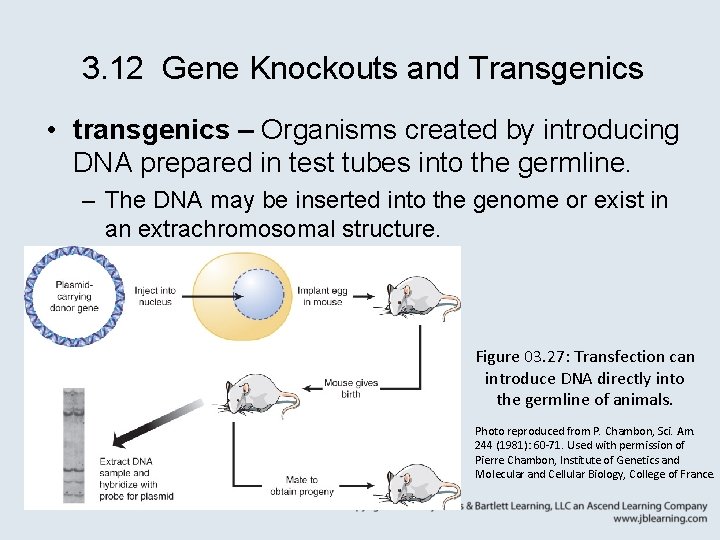
3. 12 Gene Knockouts and Transgenics • transgenics – Organisms created by introducing DNA prepared in test tubes into the germline. – The DNA may be inserted into the genome or exist in an extrachromosomal structure. Figure 03. 27: Transfection can introduce DNA directly into the germline of animals. Photo reproduced from P. Chambon, Sci. Am. 244 (1981): 60 -71. Used with permission of Pierre Chambon, Institute of Genetics and Molecular and Cellular Biology, College of France.

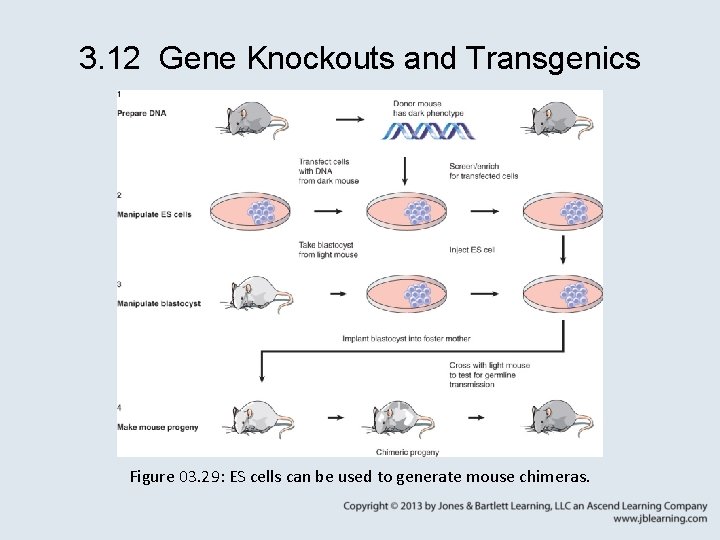
3. 12 Gene Knockouts and Transgenics • Embryonic stem (ES) cells that are injected into a mouse blastocyst generate descendant cells that become part of a chimeric adult mouse. – When the ES cells contribute to the germline, the next generation of mice may be derived from the ES cell. – Genes can be added to the mouse germline by transfecting them into ES cells before the cells are added to the blastocyst.

3. 12 Gene Knockouts and Transgenics Figure 03. 29: ES cells can be used to generate mouse chimeras.

3. 12 Gene Knockouts and Transgenics • An endogenous gene can be replaced by a transfected gene using homologous recombination. • The occurrence of successful homologous recombination can be detected by using two selectable markers, one of which is incorporated with the integrated gene, the other of which is lost when recombination occurs.

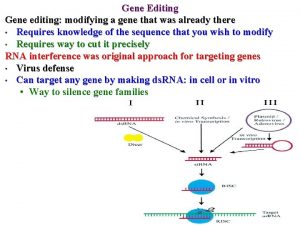
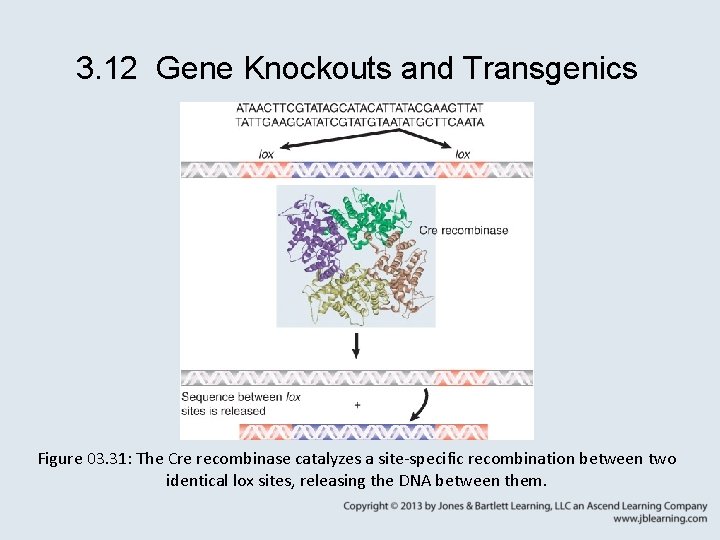
3. 12 Gene Knockouts and Transgenics • The Cre/lox system is widely used to make inducible knockouts and knock-ins. – knockout – A process in which a gene function is eliminated, usually by replacing most of the coding sequence with a selectable marker in vitro and transferring the altered gene to the genome by homologous recombination. – knock-in – A process similar to a knockout, but more subtle mutations are made.

3. 12 Gene Knockouts and Transgenics Figure 03. 31: The Cre recombinase catalyzes a site-specific recombination between two identical lox sites, releasing the DNA between them.

Chapter 15 Homologous and Site-Specific Recombination

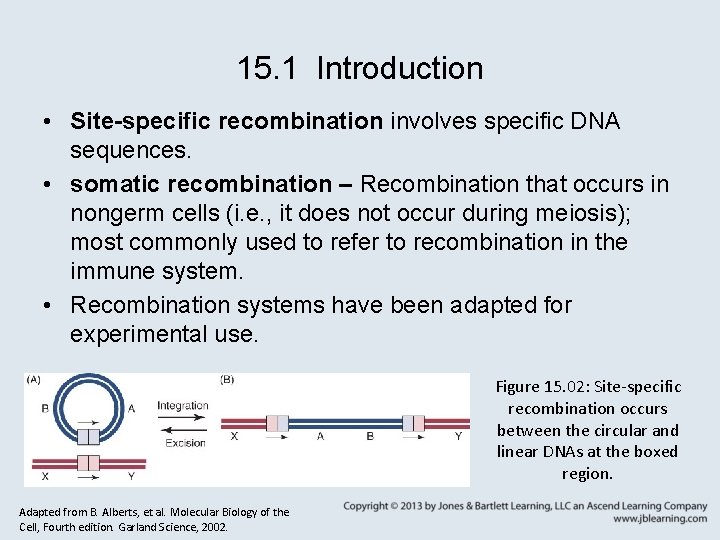
15. 1 Introduction • Homologous recombination is essential in meiosis for generating diversity and for chromosome segregation, and in mitosis to repair DNA damage and stalled replication forks. Figure 15. 01: No crossing over between the A and B genes gives rise to only nonrecombinant gametes.

15. 1 Introduction • Site-specific recombination involves specific DNA sequences. • somatic recombination – Recombination that occurs in nongerm cells (i. e. , it does not occur during meiosis); most commonly used to refer to recombination in the immune system. • Recombination systems have been adapted for experimental use. Figure 15. 02: Site-specific recombination occurs between the circular and linear DNAs at the boxed region. Adapted from B. Alberts, et al. Molecular Biology of the Cell, Fourth edition. Garland Science, 2002.

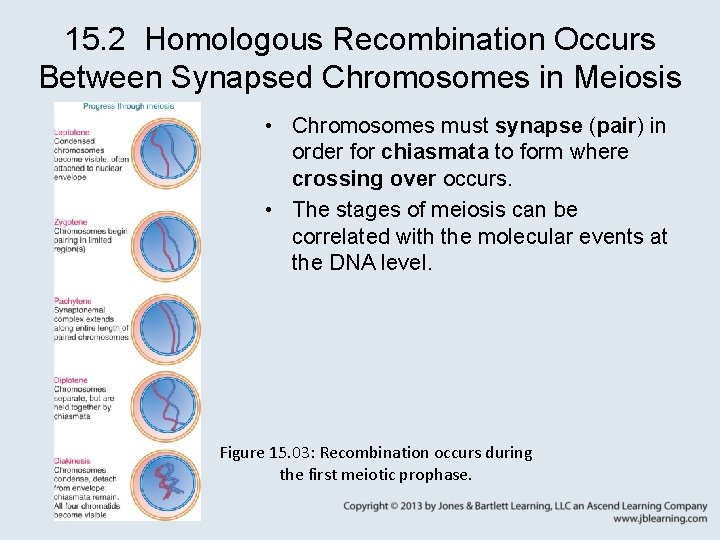
15. 2 Homologous Recombination Occurs Between Synapsed Chromosomes in Meiosis • Chromosomes must synapse (pair) in order for chiasmata to form where crossing over occurs. • The stages of meiosis can be correlated with the molecular events at the DNA level. Figure 15. 03: Recombination occurs during the first meiotic prophase.

15. 2 Homologous Recombination Occurs Between Synapsed Chromosomes in Meiosis • sister chromatid – Each of two identical copies of a replicated chromosome; this term is used as long as the two copies remain linked at the centromere. – Sister chromatids separate during anaphase in mitosis or anaphase II in meiosis. • bivalent – The structure containing all four chromatids (two representing each homolog) at the start of meiosis.

15. 2 Homologous Recombination Occurs Between Synapsed Chromosomes in Meiosis • synaptonemal complex – The morphological structure of synapsed chromosomes. • joint molecule – A pair of DNA duplexes that are connected together through a reciprocal exchange of genetic material.

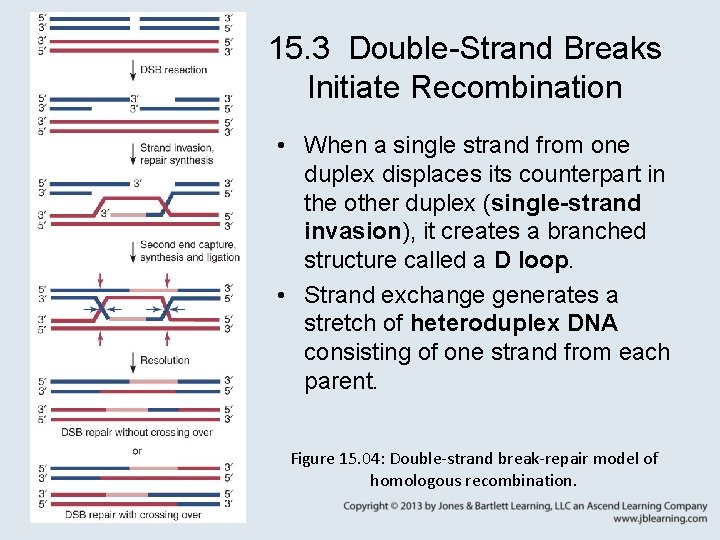
15. 3 Double-Strand Breaks Initiate Recombination • The double-strand break repair (DSBR) model of recombination is initiated by making a double-strand break in one (recipient) DNA duplex and is relevant for meiotic and mitotic homologous recombination. • In 5 end resection, exonuclease action generates 3′– single-stranded ends that invade the other (donor) duplex.

15. 3 Double-Strand Breaks Initiate Recombination • When a single strand from one duplex displaces its counterpart in the other duplex (single-strand invasion), it creates a branched structure called a D loop. • Strand exchange generates a stretch of heteroduplex DNA consisting of one strand from each parent. Figure 15. 04: Double-strand break-repair model of homologous recombination.

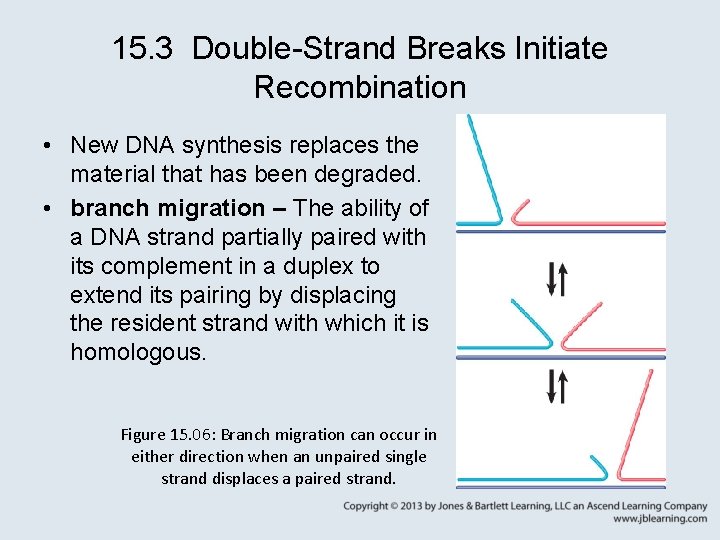
15. 3 Double-Strand Breaks Initiate Recombination • New DNA synthesis replaces the material that has been degraded. • branch migration – The ability of a DNA strand partially paired with its complement in a duplex to extend its pairing by displacing the resident strand with which it is homologous. Figure 15. 06: Branch migration can occur in either direction when an unpaired single strand displaces a paired strand.

15. 3 Double-Strand Breaks Initiate Recombination • Capture of the second DSB end by annealing generates a recombinant joint molecule in which the two DNA duplexes are connected by heteroduplex DNA and two Holliday junctions. • The joint molecule is resolved into two separate duplex molecules by nicking two of the connecting strands. • Whether recombinants are formed depends on if the strands involved in the original exchange or the other pair of strands are nicked during resolution.

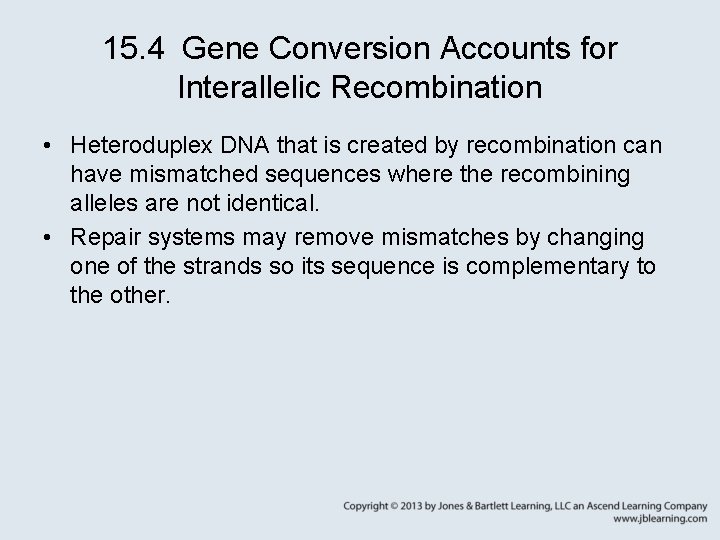
15. 4 Gene Conversion Accounts for Interallelic Recombination • Heteroduplex DNA that is created by recombination can have mismatched sequences where the recombining alleles are not identical. • Repair systems may remove mismatches by changing one of the strands so its sequence is complementary to the other.

15. 4 Gene Conversion Accounts for Interallelic Recombination • Mismatch (gap) repair of heteroduplex DNA generates nonreciprocal recombinant products called gene conversions. Figure 15. 07: Spore formation in the ascomycetes allows determination of the genetic constitution of each of the DNA strands involved in meiosis.

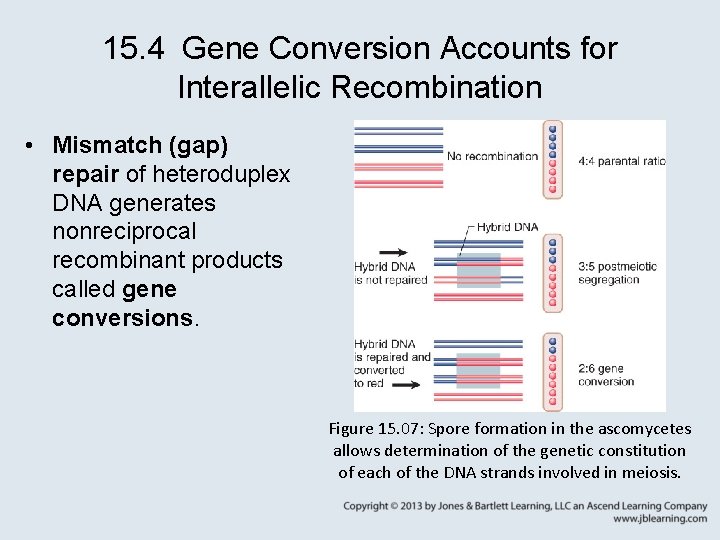
15. 5 The Synthesis. Dependent Strand. Annealing Model • The synthesis-dependent strand-annealing (SDSA) model is relevant for mitotic recombination, as it produces gene conversions from double-strand breaks without associated crossovers. Figure 15. 08: Synthesis-dependent strandannealing model of homologous recombination.

15. 6 The Single-Strand Annealing Mechanism Functions at Some Double-Strand Breaks • Single-strand annealing (SSA) occurs at doublestrand breaks between direct repeats. Figure 15. 09: Single strand annealing model of homologous recombination.

15. 6 The Single-Strand Annealing Mechanism Functions at Some Double-Strand Breaks • Resection of double-strand break ends results in 3′– single-stranded tails. • Complementarity between the repeats allows for annealing of the single strands. • The sequence between the direct repeats is deleted after SSA is completed.

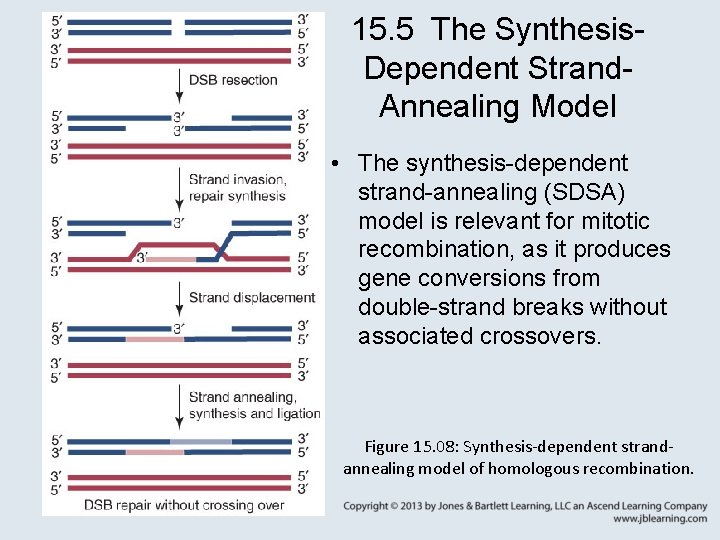
15. 7 Break-Induced Replication Can Repair Double-Strand Breaks • Break-induced replication (BIR) is initiated by a one -ended double-strand break. • BIR at repeated sequences can result in translocations. Figure 15. 10: Break-induced replication can result in nonreciprocal translocations.

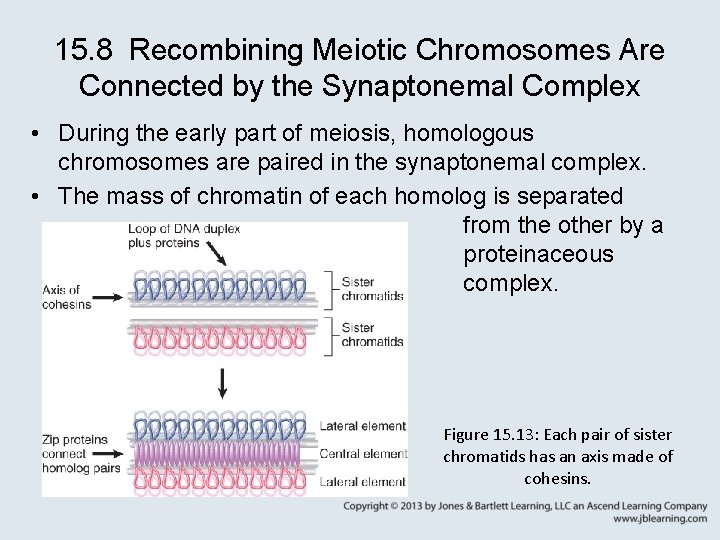
15. 8 Recombining Meiotic Chromosomes Are Connected by the Synaptonemal Complex • During the early part of meiosis, homologous chromosomes are paired in the synaptonemal complex. • The mass of chromatin of each homolog is separated from the other by a proteinaceous complex. Figure 15. 13: Each pair of sister chromatids has an axis made of cohesins.

15. 8 Recombining Meiotic Chromosomes Are Connected by the Synaptonemal Complex • axial element – A proteinaceous structure around which the chromosomes condense at the start of synapsis. • lateral element – A structure in the synaptonemal complex that forms when a pair of sister chromatids condenses on to an axial element.

15. 8 Recombining Meiotic Chromosomes Are Connected by the Synaptonemal Complex • central element – A structure that lies in the middle of the synaptonemal complex, along which the lateral elements of homologous chromosomes align. – It is formed from Zip proteins. • recombination nodules (nodes) – Dense objects present on the synaptonemal complex; they may represent protein complexes involved in crossing over.

15. 9 The Synaptonemal Complex Forms After Double-Strand Breaks • Double-strand breaks that initiate recombination occur before the synaptonemal complex forms. • If recombination is blocked, the synaptonemal complex cannot form. • Meiotic recombination involves two phases: one that results in gene conversion without crossover, and one that results in crossover products.

15. 9 The Synaptonemal Complex Forms After Double-Strand Breaks Figure 15. 15: Model of meiotic homologous recombination. Adapted from M. J. Neale and S. Keeney, Nature 442 (2006): 153 -158.

15. 10 Pairing and Synaptonemal Complex Formation Are Independent • Mutations can occur in either chromosome pairing or synaptonemal complex formation without affecting the other process.

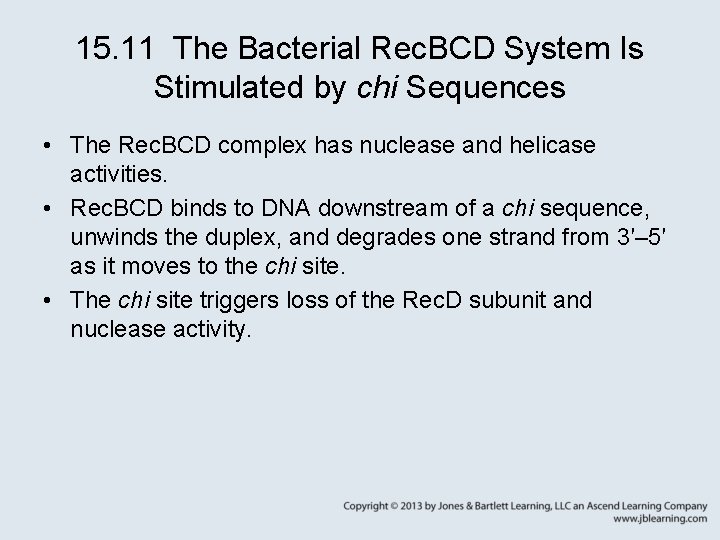
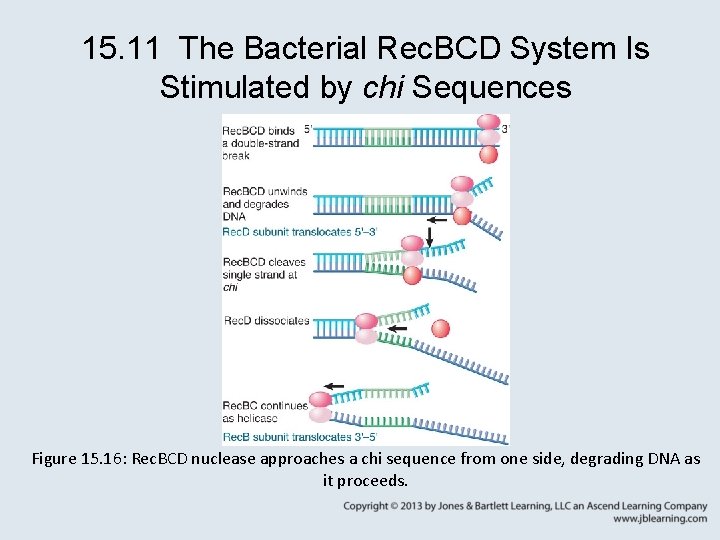
15. 11 The Bacterial Rec. BCD System Is Stimulated by chi Sequences • The Rec. BCD complex has nuclease and helicase activities. • Rec. BCD binds to DNA downstream of a chi sequence, unwinds the duplex, and degrades one strand from 3′– 5′ as it moves to the chi site. • The chi site triggers loss of the Rec. D subunit and nuclease activity.

15. 11 The Bacterial Rec. BCD System Is Stimulated by chi Sequences Figure 15. 16: Rec. BCD nuclease approaches a chi sequence from one side, degrading DNA as it proceeds.

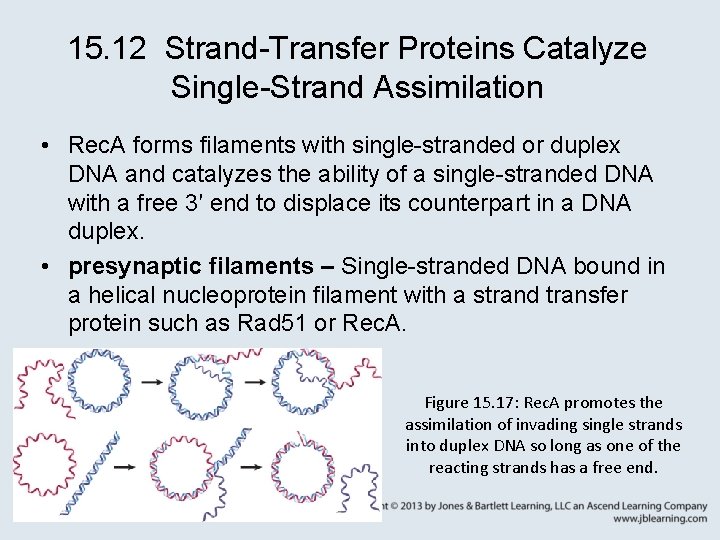
15. 12 Strand-Transfer Proteins Catalyze Single-Strand Assimilation • Rec. A forms filaments with single-stranded or duplex DNA and catalyzes the ability of a single-stranded DNA with a free 3′ end to displace its counterpart in a DNA duplex. • presynaptic filaments – Single-stranded DNA bound in a helical nucleoprotein filament with a strand transfer protein such as Rad 51 or Rec. A. Figure 15. 17: Rec. A promotes the assimilation of invading single strands into duplex DNA so long as one of the reacting strands has a free end.

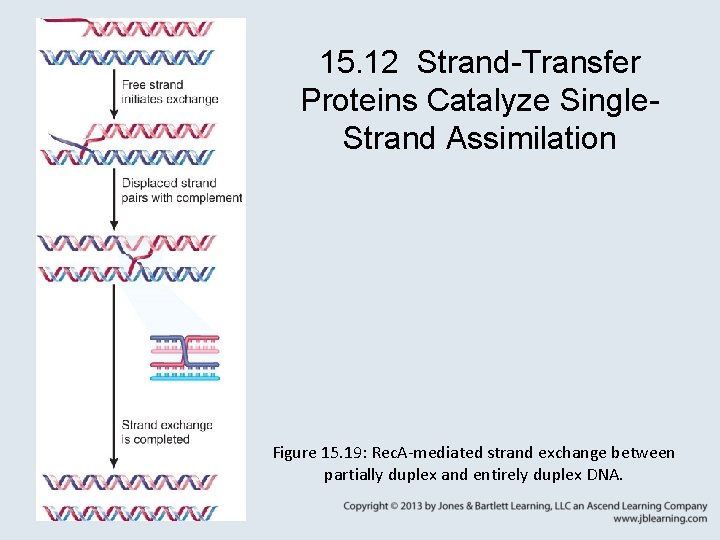
15. 12 Strand-Transfer Proteins Catalyze Single. Strand Assimilation Figure 15. 19: Rec. A-mediated strand exchange between partially duplex and entirely duplex DNA.

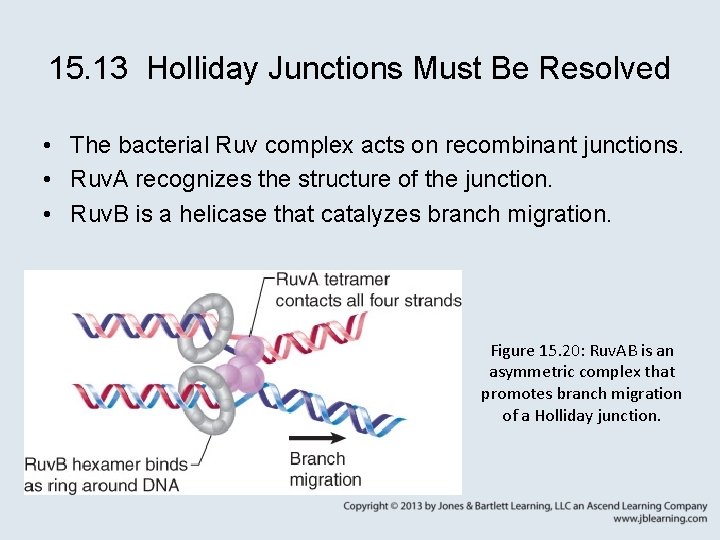
15. 13 Holliday Junctions Must Be Resolved • The bacterial Ruv complex acts on recombinant junctions. • Ruv. A recognizes the structure of the junction. • Ruv. B is a helicase that catalyzes branch migration. Figure 15. 20: Ruv. AB is an asymmetric complex that promotes branch migration of a Holliday junction.

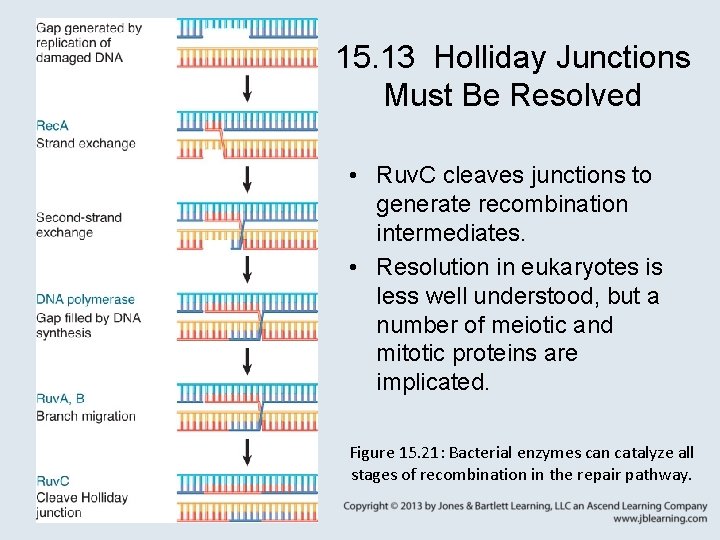
15. 13 Holliday Junctions Must Be Resolved • Ruv. C cleaves junctions to generate recombination intermediates. • Resolution in eukaryotes is less well understood, but a number of meiotic and mitotic proteins are implicated. Figure 15. 21: Bacterial enzymes can catalyze all stages of recombination in the repair pathway.

15. 13 Holliday Junctions Must Be Resolved • patch recombinant – DNA that results from a Holliday junction being resolved by cutting the exchanged strands. – The duplex is largely unchanged, except for a DNA sequence on one strand that came from the homologous chromosome.

15. 13 Holliday Junctions Must Be Resolved • splice recombinant – DNA that results from a Holliday junction being resolved by cutting the nonexchanged strands. – Both strands of DNA before the exchange point come from one chromosome; the DNA after the exchange point come from the homologous chromosome.

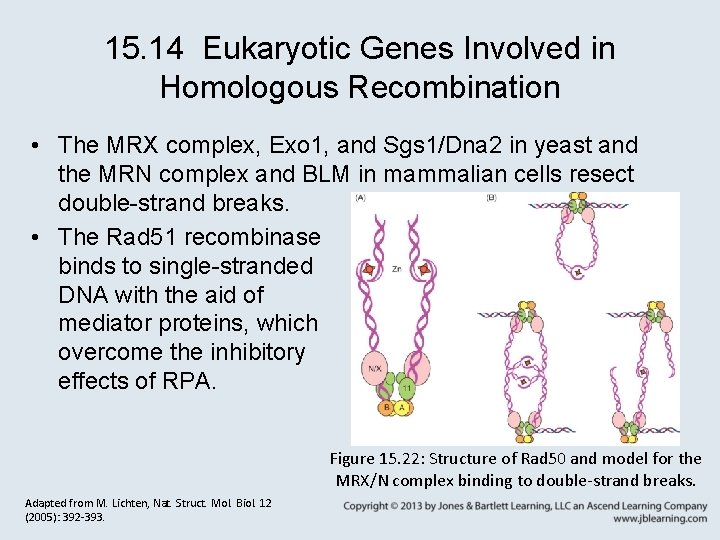
15. 14 Eukaryotic Genes Involved in Homologous Recombination • The MRX complex, Exo 1, and Sgs 1/Dna 2 in yeast and the MRN complex and BLM in mammalian cells resect double-strand breaks. • The Rad 51 recombinase binds to single-stranded DNA with the aid of mediator proteins, which overcome the inhibitory effects of RPA. Figure 15. 22: Structure of Rad 50 and model for the MRX/N complex binding to double-strand breaks. Adapted from M. Lichten, Nat. Struct. Mol. Biol. 12 (2005): 392 -393.

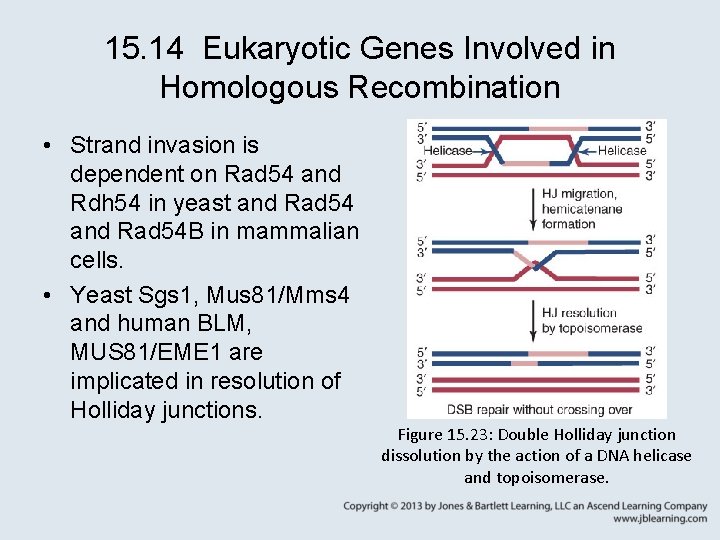
15. 14 Eukaryotic Genes Involved in Homologous Recombination • Strand invasion is dependent on Rad 54 and Rdh 54 in yeast and Rad 54 B in mammalian cells. • Yeast Sgs 1, Mus 81/Mms 4 and human BLM, MUS 81/EME 1 are implicated in resolution of Holliday junctions. Figure 15. 23: Double Holliday junction dissolution by the action of a DNA helicase and topoisomerase.

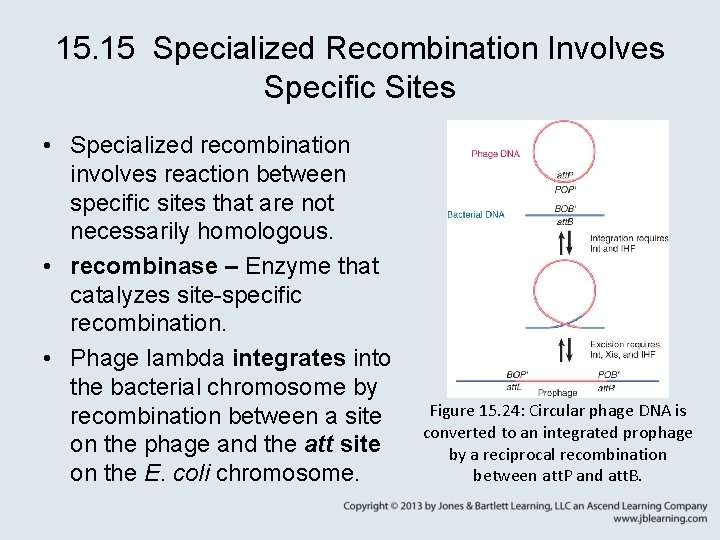
15. 15 Specialized Recombination Involves Specific Sites • Specialized recombination involves reaction between specific sites that are not necessarily homologous. • recombinase – Enzyme that catalyzes site-specific recombination. • Phage lambda integrates into the bacterial chromosome by recombination between a site on the phage and the att site on the E. coli chromosome. Figure 15. 24: Circular phage DNA is converted to an integrated prophage by a reciprocal recombination between att. P and att. B.

15. 15 Specialized Recombination Involves Specific Sites • core sequence – The segment of DNA that is common to the attachment sites on both the phage lambda and bacterial genomes. – It is the location of the recombination event that allows phage lambda to integrate. • The phage is excised from the chromosome by recombination between the sites at the end of the linear prophage. • Phage lambda int codes for an integrase that catalyzes the integration reaction.

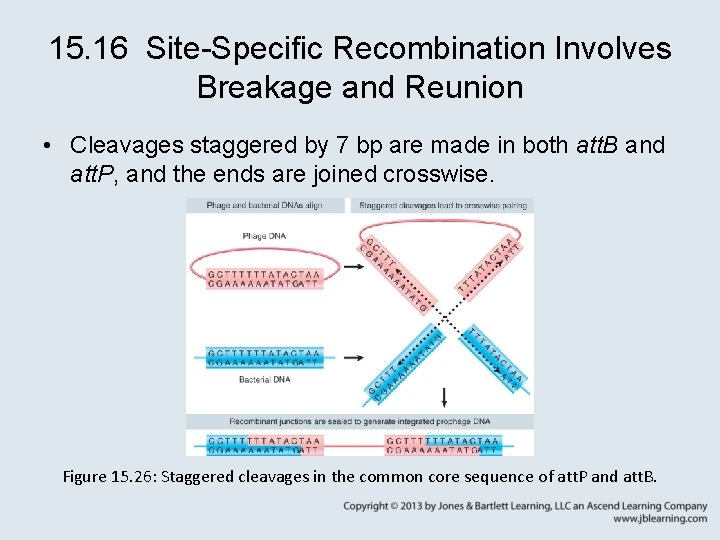
15. 16 Site-Specific Recombination Involves Breakage and Reunion • Cleavages staggered by 7 bp are made in both att. B and att. P, and the ends are joined crosswise. Figure 15. 26: Staggered cleavages in the common core sequence of att. P and att. B.

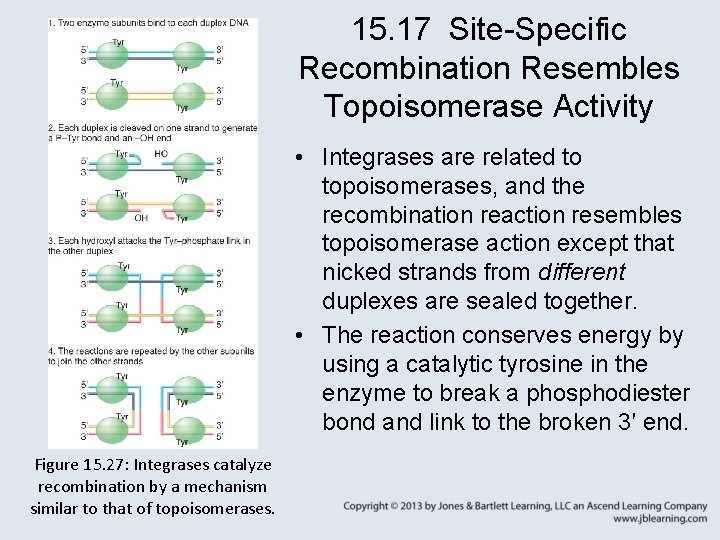
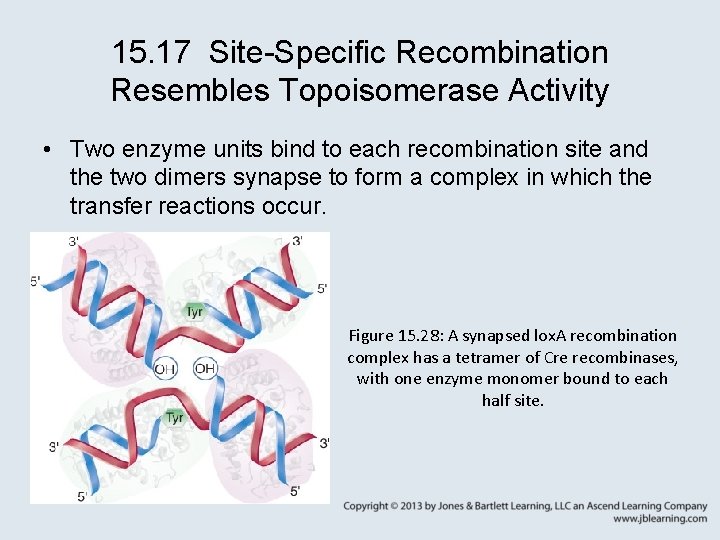
15. 17 Site-Specific Recombination Resembles Topoisomerase Activity • Integrases are related to topoisomerases, and the recombination reaction resembles topoisomerase action except that nicked strands from different duplexes are sealed together. • The reaction conserves energy by using a catalytic tyrosine in the enzyme to break a phosphodiester bond and link to the broken 3′ end. Figure 15. 27: Integrases catalyze recombination by a mechanism similar to that of topoisomerases.

15. 17 Site-Specific Recombination Resembles Topoisomerase Activity • Two enzyme units bind to each recombination site and the two dimers synapse to form a complex in which the transfer reactions occur. Figure 15. 28: A synapsed lox. A recombination complex has a tetramer of Cre recombinases, with one enzyme monomer bound to each half site.

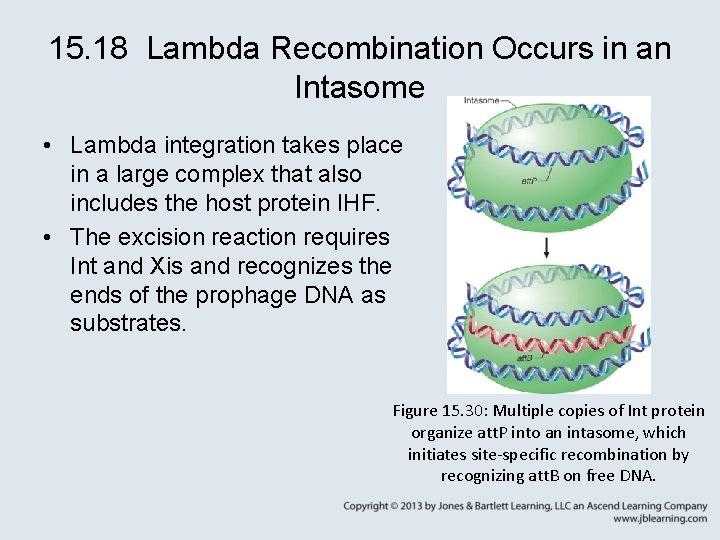
15. 18 Lambda Recombination Occurs in an Intasome • Lambda integration takes place in a large complex that also includes the host protein IHF. • The excision reaction requires Int and Xis and recognizes the ends of the prophage DNA as substrates. Figure 15. 30: Multiple copies of Int protein organize att. P into an intasome, which initiates site-specific recombination by recognizing att. B on free DNA.

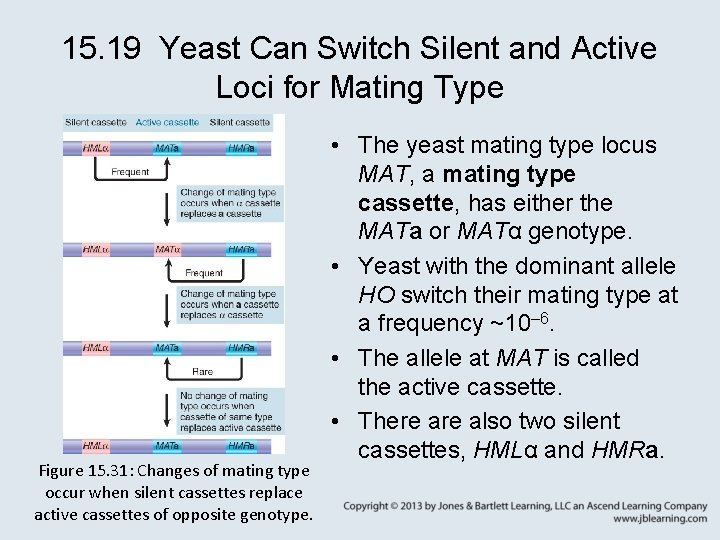
15. 19 Yeast Can Switch Silent and Active Loci for Mating Type Figure 15. 31: Changes of mating type occur when silent cassettes replace active cassettes of opposite genotype. • The yeast mating type locus MAT, a mating type cassette, has either the MATa or MATα genotype. • Yeast with the dominant allele HO switch their mating type at a frequency ~10– 6. • The allele at MAT is called the active cassette. • There also two silent cassettes, HMLα and HMRa.

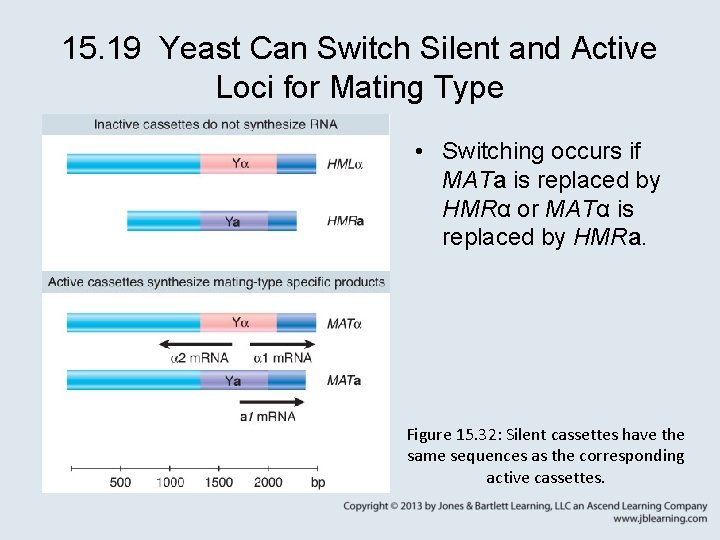
15. 19 Yeast Can Switch Silent and Active Loci for Mating Type • Switching occurs if MATa is replaced by HMRα or MATα is replaced by HMRa. Figure 15. 32: Silent cassettes have the same sequences as the corresponding active cassettes.

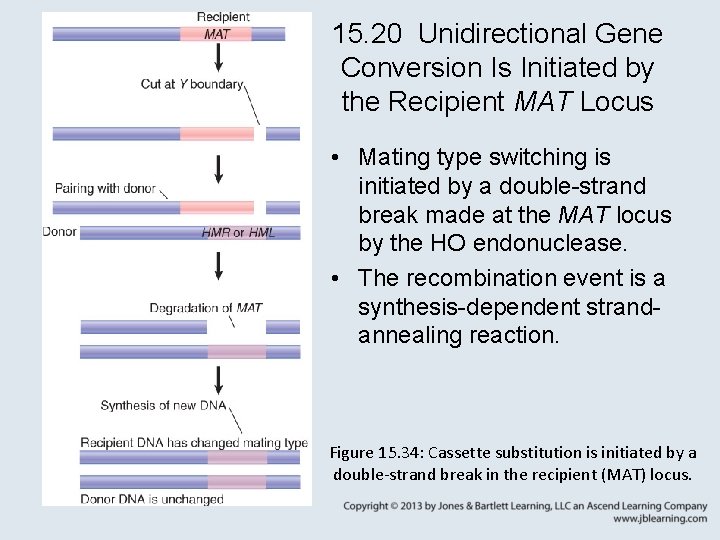
15. 20 Unidirectional Gene Conversion Is Initiated by the Recipient MAT Locus • Mating type switching is initiated by a double-strand break made at the MAT locus by the HO endonuclease. • The recombination event is a synthesis-dependent strandannealing reaction. Figure 15. 34: Cassette substitution is initiated by a double-strand break in the recipient (MAT) locus.

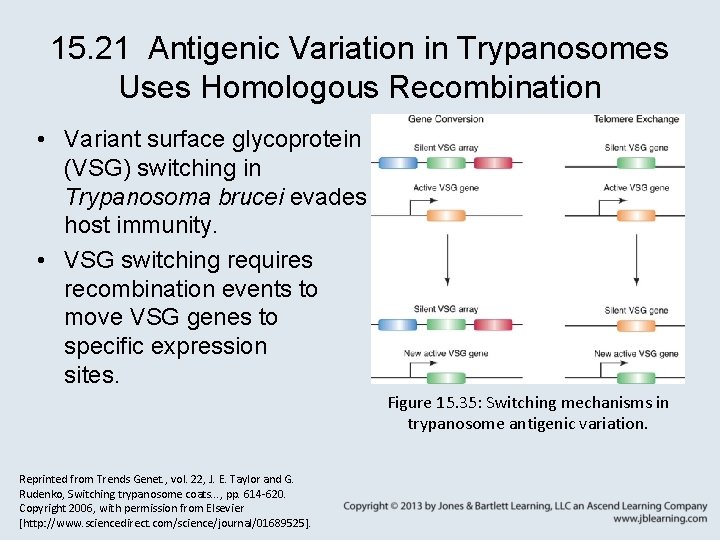
15. 21 Antigenic Variation in Trypanosomes Uses Homologous Recombination • Variant surface glycoprotein (VSG) switching in Trypanosoma brucei evades host immunity. • VSG switching requires recombination events to move VSG genes to specific expression sites. Figure 15. 35: Switching mechanisms in trypanosome antigenic variation. Reprinted from Trends Genet. , vol. 22, J. E. Taylor and G. Rudenko, Switching trypanosome coats. . . , pp. 614 -620. Copyright 2006, with permission from Elsevier [http: //www. sciencedirect. com/science/journal/01689525].

15. 22 Recombination Pathways Adapted for Experimental Systems • Mitotic homologous recombination allows for targeted transformation. • The Cre/lox and Flp/FRT systems allow for targeted recombination and gene knockout construction.

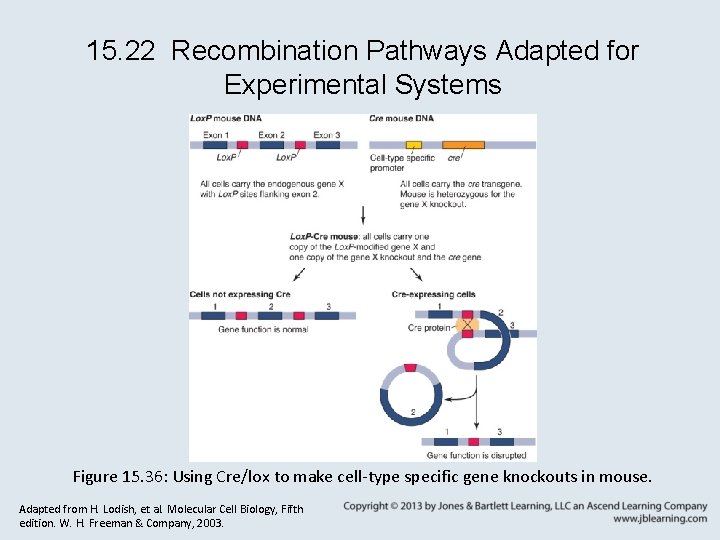
15. 22 Recombination Pathways Adapted for Experimental Systems Figure 15. 36: Using Cre/lox to make cell-type specific gene knockouts in mouse. Adapted from H. Lodish, et al. Molecular Cell Biology, Fifth edition. W. H. Freeman & Company, 2003.

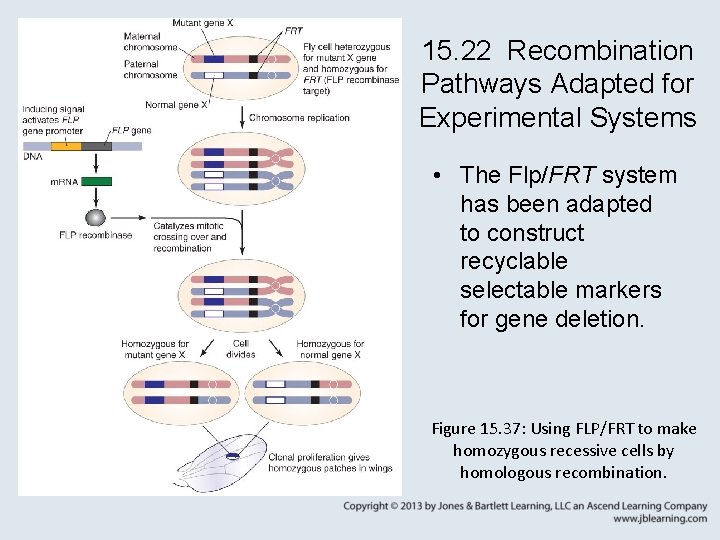
15. 22 Recombination Pathways Adapted for Experimental Systems • The Flp/FRT system has been adapted to construct recyclable selectable markers for gene deletion. Figure 15. 37: Using FLP/FRT to make homozygous recessive cells by homologous recombination.
 Knockouts
Knockouts Single celled and multicellular organisms
Single celled and multicellular organisms Gene by gene test results
Gene by gene test results Chapter 17 gene expression from gene to protein
Chapter 17 gene expression from gene to protein Why do organisms interact with other organisms
Why do organisms interact with other organisms Section 4 gene regulation and mutations
Section 4 gene regulation and mutations Section 3 gene linkage and polyploidy
Section 3 gene linkage and polyploidy The founder effect
The founder effect What is gene flow and genetic drift
What is gene flow and genetic drift Section 11-3 exploring mendelian genetics answer key
Section 11-3 exploring mendelian genetics answer key Gene and chromosome
Gene and chromosome What is the difference between genetic drift and gene flow
What is the difference between genetic drift and gene flow Wikipedia
Wikipedia Genetic drift vs genetic flow
Genetic drift vs genetic flow Chapter 12 section 4: gene regulation and mutations
Chapter 12 section 4: gene regulation and mutations Section 11-5 linkage and gene maps
Section 11-5 linkage and gene maps Mendel and the gene idea chapter 14
Mendel and the gene idea chapter 14 Chapter 14 mendel and the gene idea
Chapter 14 mendel and the gene idea Chapter 10 section 3 gene linkage and polyploidy
Chapter 10 section 3 gene linkage and polyploidy Section 11-5 linkage and gene maps answer key
Section 11-5 linkage and gene maps answer key Gene prediction in prokaryotes and eukaryotes
Gene prediction in prokaryotes and eukaryotes Lac operon inducible or repressible
Lac operon inducible or repressible Chapter 14 mendel and the gene idea
Chapter 14 mendel and the gene idea Chapter 14 mendel and the gene idea
Chapter 14 mendel and the gene idea Chapter 14 mendel and the gene idea
Chapter 14 mendel and the gene idea Chapter 14 mendel and the gene idea
Chapter 14 mendel and the gene idea Taxonomic hierarchy of cat
Taxonomic hierarchy of cat Section 1 organisms and their relationships
Section 1 organisms and their relationships Blue things
Blue things Organisms and the environment
Organisms and the environment Principles of ecology section 3 cycling of matter
Principles of ecology section 3 cycling of matter Principles of ecology section 2 flow of energy
Principles of ecology section 2 flow of energy Chapter 2 principles of ecology
Chapter 2 principles of ecology Principles of ecology chapter 2
Principles of ecology chapter 2 Unicellular vs multicellular
Unicellular vs multicellular Zones in lake ecosystem and types of organisms present
Zones in lake ecosystem and types of organisms present How are organisms classified into domains and kingdoms
How are organisms classified into domains and kingdoms 5 levels of organisms
5 levels of organisms Singlets needle leaves
Singlets needle leaves How are unicellular and multicellular organisms alike
How are unicellular and multicellular organisms alike In the hierarchy of classification which grouping
In the hierarchy of classification which grouping Diagram of unicellular organism
Diagram of unicellular organism Unicellular examples
Unicellular examples Single celled life form
Single celled life form This is the study of grouping and naming organisms
This is the study of grouping and naming organisms The scientific discipline of classifying organisms
The scientific discipline of classifying organisms What is wrong with this
What is wrong with this Discipline of classifying and naming organisms
Discipline of classifying and naming organisms The science of naming and classifying organisms
The science of naming and classifying organisms Class order family genus species
Class order family genus species Chapter 2 section 1 organisms and their relationships
Chapter 2 section 1 organisms and their relationships Discipline of classifying and naming organisms
Discipline of classifying and naming organisms