10 4 Energy Levels of Electrons Electrons move



















![Example for you to try • You try Ba 2 • Ba = [Xe] Example for you to try • You try Ba 2 • Ba = [Xe]](https://slidetodoc.com/presentation_image_h2/196322404eacee79071414ec38f34c30/image-25.jpg)
- Slides: 25

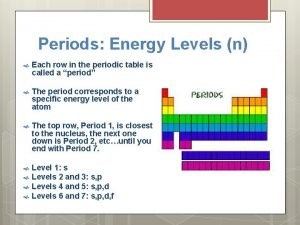
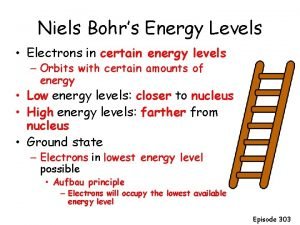
10. 4 Energy Levels of Electrons • Electrons move in definite energy levels; these are labeled 1 - 7 • Each level has sublevel(s) which are probability shapes that show where the electrons may be at any one time. Also known as orbitals. • S orbital can hold up to 2 electrons (0, 1, 2) • p orbital can hold up to 6 electrons (0 -6) • d orbital can hold up to 10 electrons (0 -10) • f orbital can hold up to 14 electrons (0 -14) • Aufbau chart shows how electrons fill into the main energy levels and the sublevels or orbitals

Energy Levels and Sublevels • • 1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f

Aufbau Diagram or Chart • • 1 s 2 s 3 s 4 s 5 s 6 s 7 s START HERE and follow 2 p the arrows! 3 p 3 d 4 p 4 d 4 f 5 p 5 d 5 f 6 p 6 d 6 f 7 p 7 d 7 f

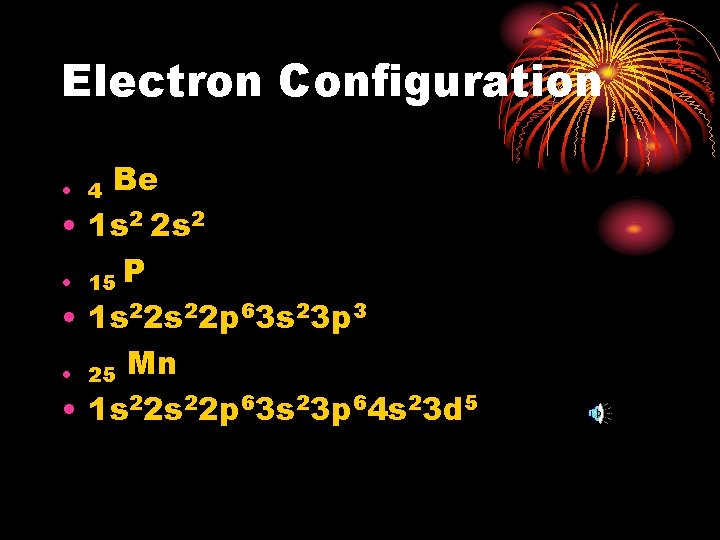
Electron Configuration Be • 1 s 2 2 s 2 • 15 P • 1 s 22 p 63 s 23 p 3 • 25 Mn • 1 s 22 p 63 s 23 p 64 s 23 d 5 • 4

Pauli Exclusion Principle • Pauli exclusion principle states that no more than 2 electrons can be in the same suborbital. Even so, this would cause them to have precisely the same quantum address. So Pauli decided there has to be a way to tell one electron from another. In other words, they must differ by at least one quantum number!

Pauli Exclusion Principle • So they invented spin (+1/2 or -1/2) called spin up and spin down. Has nothing to do with the direction of the electron--we don’ t know how they move just where they may be at with 90% chance of finding it inside the energy level and orbital designated.

Hund’s Rule • Hund’s rule states that electrons fill unpaired until there is no more room then they will pair (applies to p, d and f orbitals)

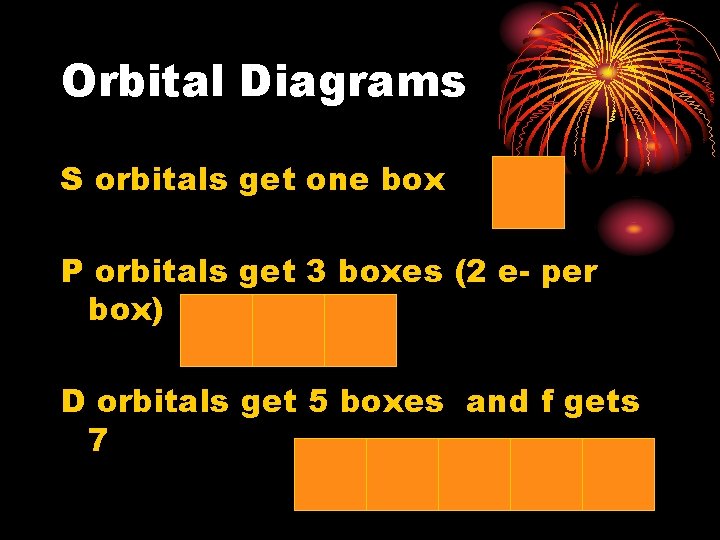
Orbital Diagrams S orbitals get one box P orbitals get 3 boxes (2 e- per box) D orbitals get 5 boxes and f gets 7

Orbital Diagrams (cont. ) • Insert electrons (using arrows into each box according to Hund’s and Pauli) 2 p 3

Answer • 2 p 3 (arrows can all point up or down) • Now try 4 f 10

Answer to 10 4 f • Arrows may point up or down if they are in boxes individually; however, if there are 2 electrons in a box, one must point up and one down.

Electrons and the Periodic Table Revisited History of the Table Periodic Law Important People

Mendeleev • Mendeleev was a Russian chemist who arranged the known elements in vertical columns in order of increasing mass and noticed a pattern in physical and chemical properties

Mosley • Mosley was a British physicist who determined the atomic number (number of protons) of the atoms of elements and then arranged the elements according to their atomic number.

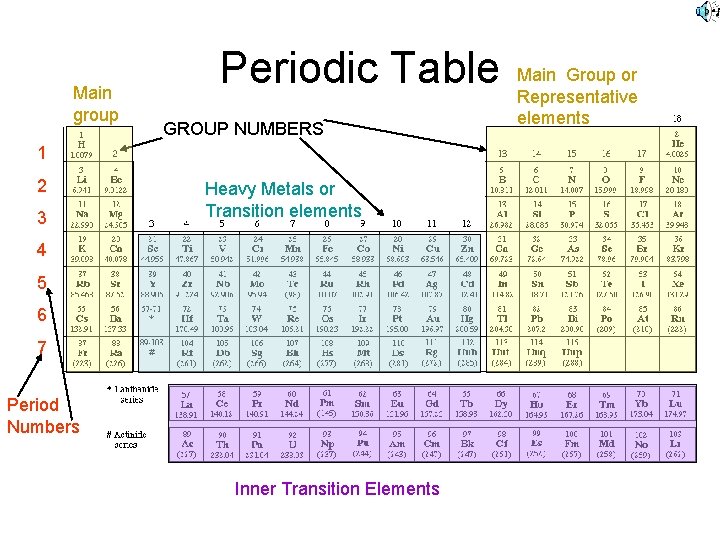
Periods and Groups • Periods of the periodic table are the rows across • Groups or Families are columns on the periodic table. • Currently we have 18 groups. We will use the 1 -18 designations not the A/B or Roman Numerals

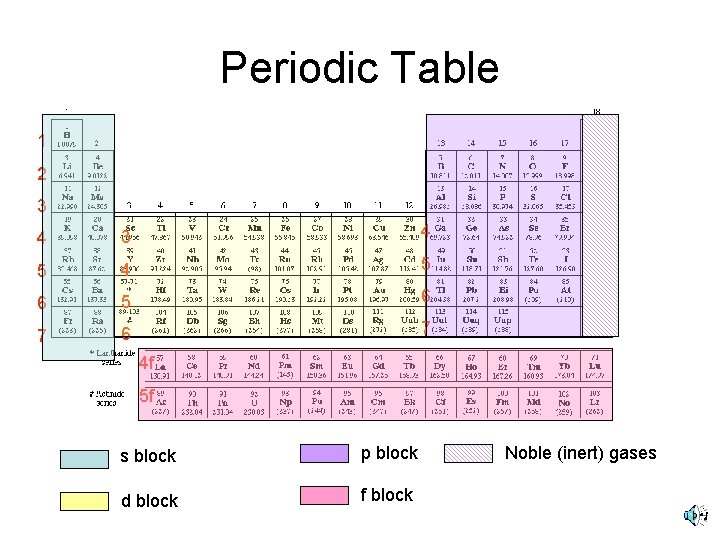
Areas of the Periodic Table • Representative elements or Main Group are those that are in Groups 1, 2, 13, 14, 15, 16, 17, 18 • Transition elements are Groups 3 - 12 , also called the Heavy Metals

Inner Transition • Rare Earth elements that are located in the bottom two rows (away from the rest of the table) of the periodic table

Main group Periodic Table GROUP NUMBERS 1 2 3 Heavy Metals or Transition elements 4 5 6 7 Period Numbers Inner Transition Elements Main Group or Representative elements

Groups with names • • Group 1 = Alkali Metals 2 = Alkaline Earth Metals 18 = Inert or Noble Gases 17 = Halogens

Periodic Table and Electron Configuration • The light metals compose the s block. • The transition elements are the d block. • The nonmetals are p block. • The inner transition (rare earth) metals are the f block.

Periodic Table 1 2 3 4 5 4 5 6 7 6 7 4 f 5 f s block p block d block f block Noble (inert) gases

• • Complete the electron configurations for the Noble Gases (Hint: Group 18) • Xe = He 1 s 2 Ne 1 s 22 p 6 Ar = 1 s 22 p 63 s 23 p 6 Kr = 1 s 22 p 63 s 23 p 64 s 23 d 1 04 p 6 1 s 22 p 63 s 23 p 64 s 23 d 10 4 p 65 s 24 d 105 p 6 • Rn = 1 s 22 p 63 s 23 p 64 s 23 d 10 4 p 65 s 24 d 105 p 66 s 24 f 145 d 1 06 p 6 Except He, do you see a trend in all of the noble gas configurations? • What do they all end in?

Shorthand Notation • We use the noble gases in shorthand notation • Find the closest noble gas that has an atomic number LESS than that of the element

Example • • Ex. K What is K’s atomic number? 19 Closest noble gas? Ar What is Ar’s atomic number? 18 = 1 s 22 p 63 s 23 p 6 = [Ar] 4 s 1 = Means the first 18 electrons are arranged like argon and the last electron is called the VALENCE ELECTRON (outermost shell)
![Example for you to try You try Ba 2 Ba Xe Example for you to try • You try Ba 2 • Ba = [Xe]](https://slidetodoc.com/presentation_image_h2/196322404eacee79071414ec38f34c30/image-25.jpg)
Example for you to try • You try Ba 2 • Ba = [Xe] 6 s • Try Pb • Pb = [Xe] 6 s 24 f 145 d 106 p 2
 Sublevels
Sublevels Lesson 1 electrons and energy levels
Lesson 1 electrons and energy levels Lesson 1 electrons and energy levels
Lesson 1 electrons and energy levels What has roads but no cars rivers but no water
What has roads but no cars rivers but no water Lesson 27 electrons on the move answers
Lesson 27 electrons on the move answers How many levels are there in costa’s levels of thinking?
How many levels are there in costa’s levels of thinking? Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis How does energy move through the biosphere
How does energy move through the biosphere Do birds eat squirrels
Do birds eat squirrels How does energy move
How does energy move Maximum elastic potential energy
Maximum elastic potential energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy How many electrons are in the third energy level?
How many electrons are in the third energy level? High energy electrons
High energy electrons Energy in trophic levels
Energy in trophic levels Principal energy levels
Principal energy levels Periodic table energy levels
Periodic table energy levels How do you find the energy level of an element
How do you find the energy level of an element Food web energy flow
Food web energy flow Energy levels periodic table
Energy levels periodic table Electrons in an atom's outermost orbitals are called
Electrons in an atom's outermost orbitals are called How to read electron configuration
How to read electron configuration Energy levels
Energy levels Ecology pyramid
Ecology pyramid