Quantum Theory and Electron Configuration Electrons do not














- Slides: 52

Quantum Theory and Electron Configuration

Electrons do not move in orbits! • Erwin Schrödinger (1887 -1961) improved on Bohr’s theory by creating an equation describing the location and the energy of an electron in a hydrogen atom (1926). • The description of how electrons behave in atoms comes from the mechanical solution to the Schrodinger equations – the quantum mechanical model.

Quantum Mechanics • Quantum mechanics (wave mechanics) are used only for the motion of subatomic particles; they cannot be used for larger quantities of matter. • Schrodinger’s work was aided by another German physicist, Werner Heisenberg (1901 -1976), who stated his now famous uncertainty principle – it is impossible to know both the precise location and precise velocity at the same time. • Why is this true?

The Uncertainty Principle • In order to observe a particle, we must interact with it. • We may use photons of light to “see” the particle. • But the photon, which has mass and energy, would interact with the particle and change the velocity. • So it is impossible to be sure of the velocity at the moment of observation.

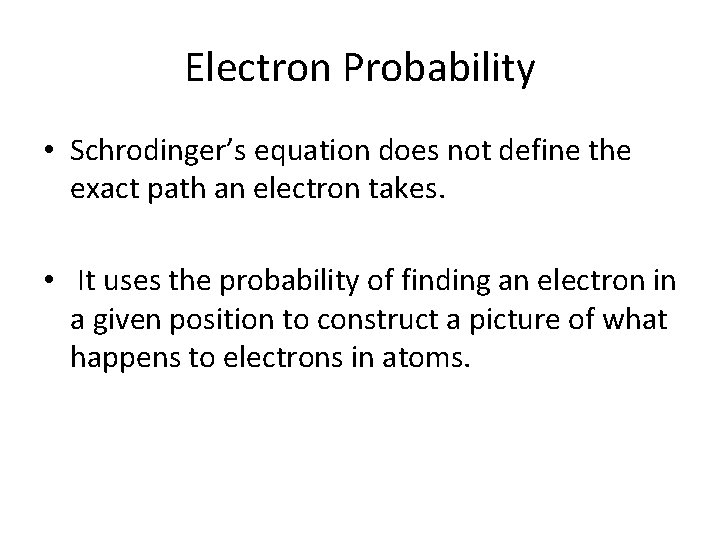
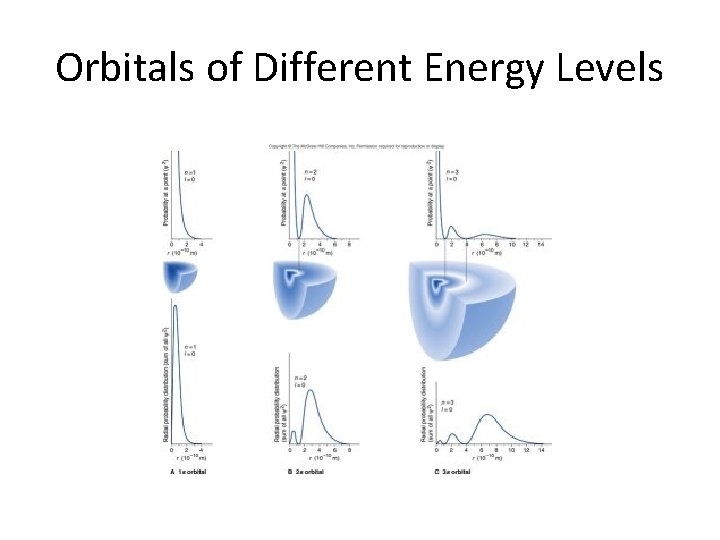
Electron Probability • Schrodinger’s equation does not define the exact path an electron takes. • It uses the probability of finding an electron in a given position to construct a picture of what happens to electrons in atoms.

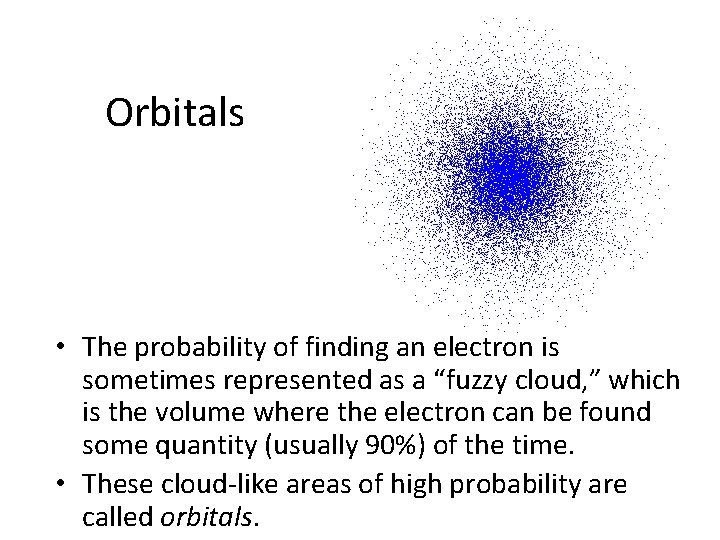
Orbitals • The probability of finding an electron is sometimes represented as a “fuzzy cloud, ” which is the volume where the electron can be found some quantity (usually 90%) of the time. • These cloud-like areas of high probability are called orbitals.

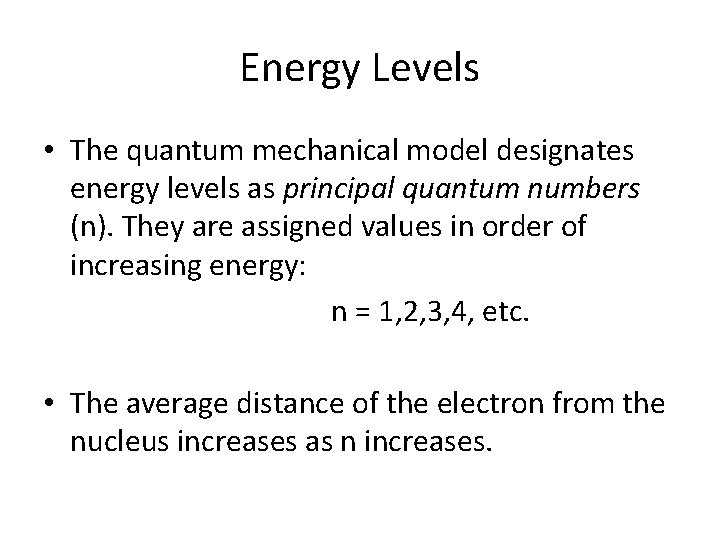
Energy Levels • The quantum mechanical model designates energy levels as principal quantum numbers (n). They are assigned values in order of increasing energy: n = 1, 2, 3, 4, etc. • The average distance of the electron from the nucleus increases as n increases.

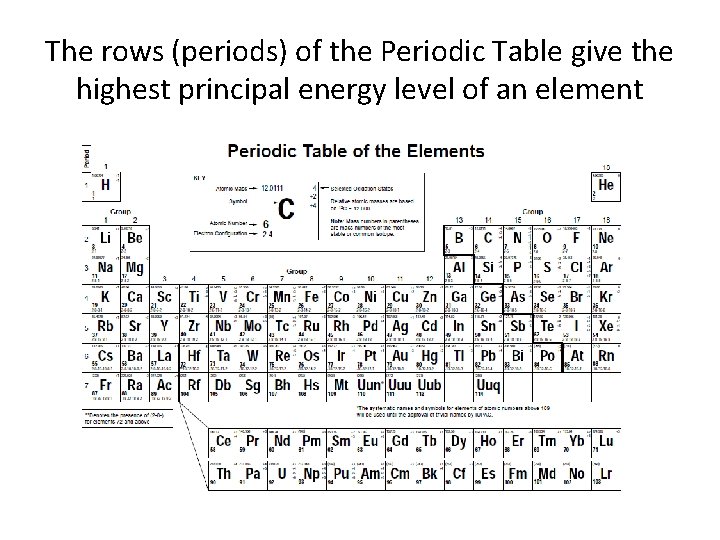
The rows (periods) of the Periodic Table give the highest principal energy level of an element

Orbitals of Different Energy Levels

Energy Levels and Sublevels • Within each principal energy level, electrons occupy sublevels of energy, the number of which varies. • The sublevels exist because the electrons have complex “social” relationships with each other. • The number of sublevels is identical to the principal quantum number.


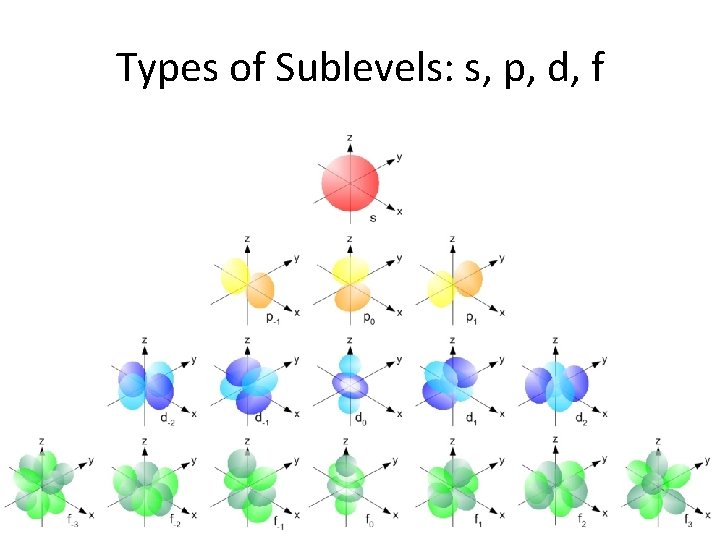
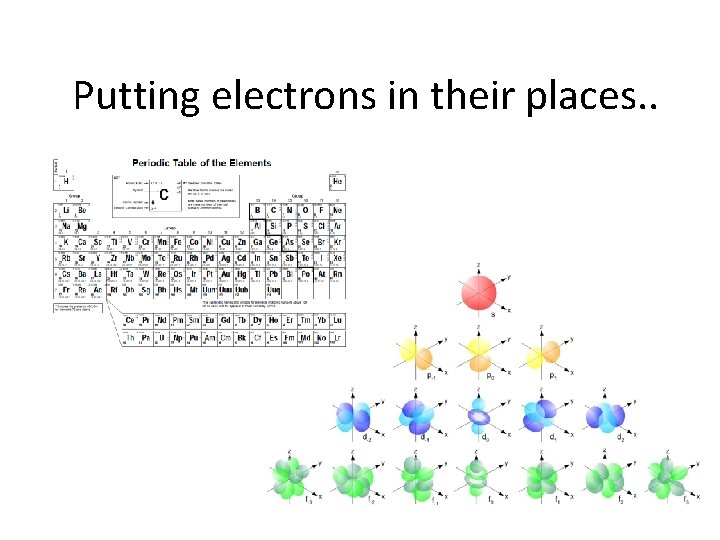
What about the paths of the electrons? • The cloud-like probability shapes are called orbitals. Why can’t they be called orbits? • The orbitals are contained within sublevels, and are given letter designations: s, p, d, f

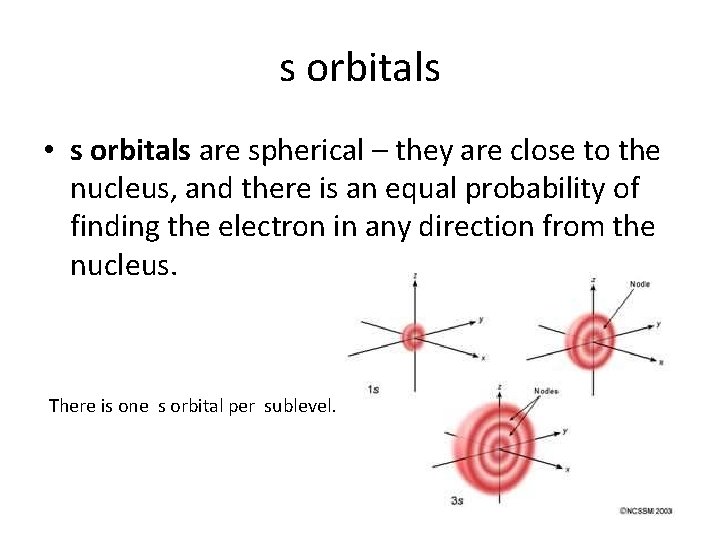
s orbitals • s orbitals are spherical – they are close to the nucleus, and there is an equal probability of finding the electron in any direction from the nucleus. There is one s orbital per sublevel.

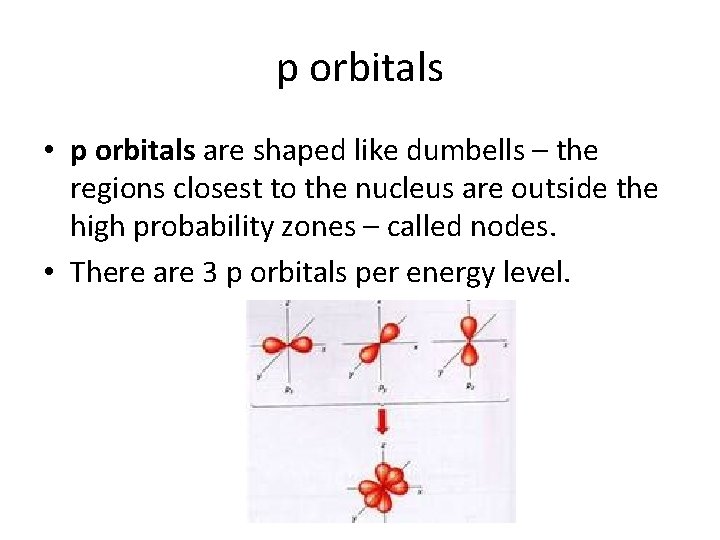
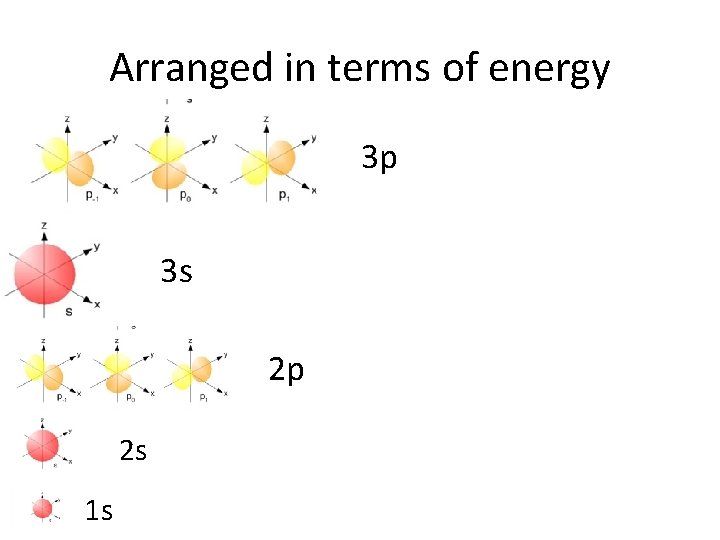
p orbitals • p orbitals are shaped like dumbells – the regions closest to the nucleus are outside the high probability zones – called nodes. • There are 3 p orbitals per energy level.

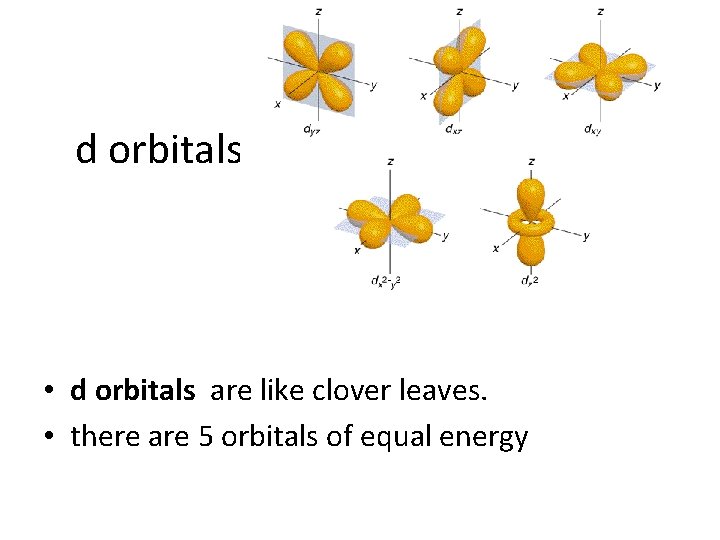
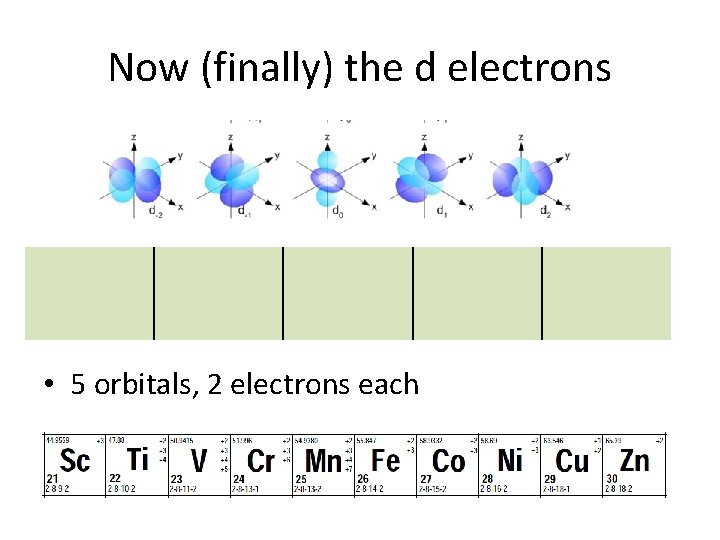
d orbitals • d orbitals are like clover leaves. • there are 5 orbitals of equal energy

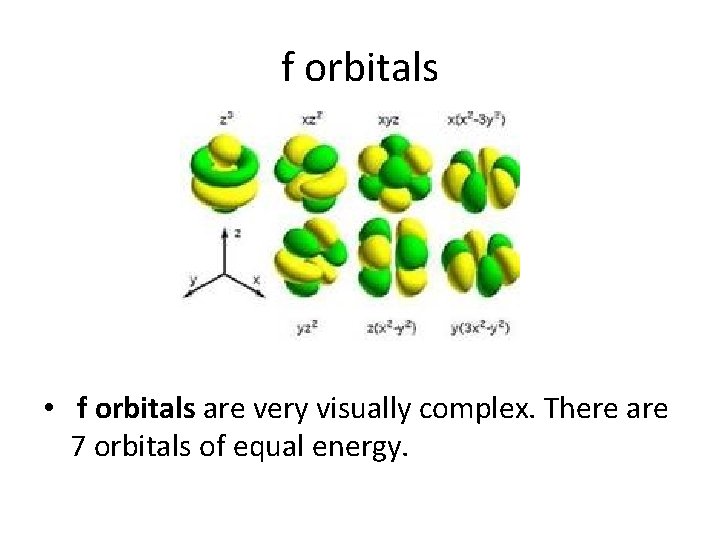
f orbitals • f orbitals are very visually complex. There are 7 orbitals of equal energy.

Types of Sublevels: s, p, d, f


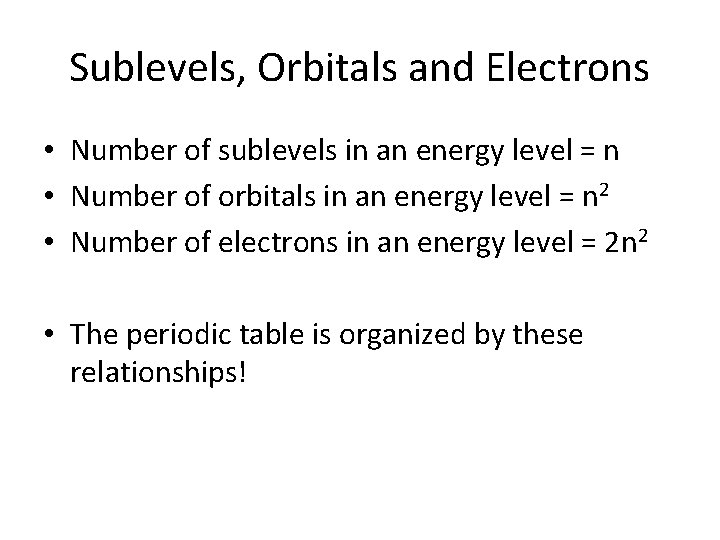
Sublevels, Orbitals and Electrons • Number of sublevels in an energy level = n • Number of orbitals in an energy level = n 2 • Number of electrons in an energy level = 2 n 2 • The periodic table is organized by these relationships!

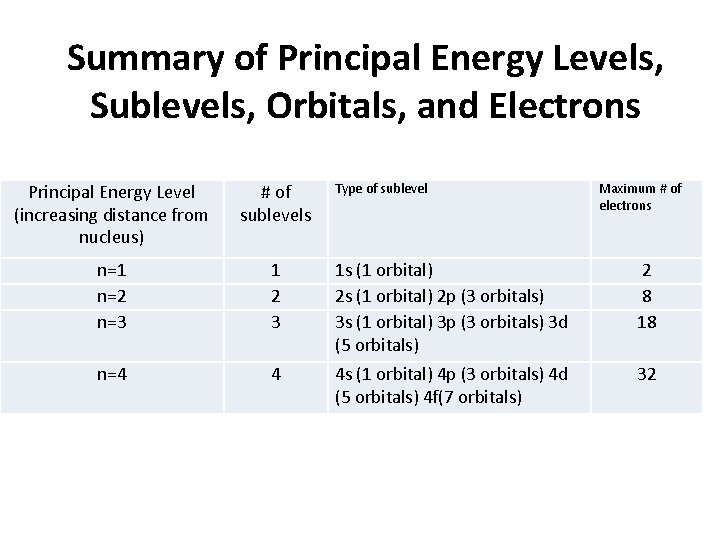
Summary of Principal Energy Levels, Sublevels, Orbitals, and Electrons Principal Energy Level (increasing distance from nucleus) # of sublevels n=1 n=2 n=3 1 2 3 1 s (1 orbital) 2 p (3 orbitals) 3 s (1 orbital) 3 p (3 orbitals) 3 d (5 orbitals) 2 8 18 n=4 4 4 s (1 orbital) 4 p (3 orbitals) 4 d (5 orbitals) 4 f(7 orbitals) 32 Type of sublevel Maximum # of electrons


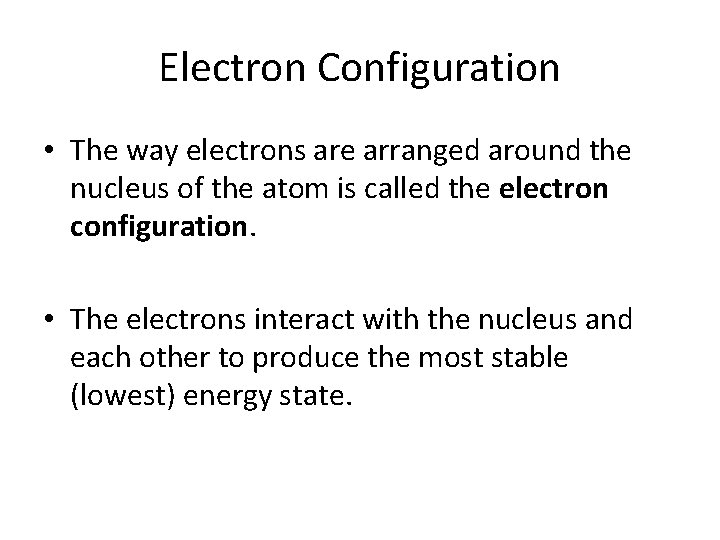
Electron Configuration • The way electrons are arranged around the nucleus of the atom is called the electron configuration. • The electrons interact with the nucleus and each other to produce the most stable (lowest) energy state.

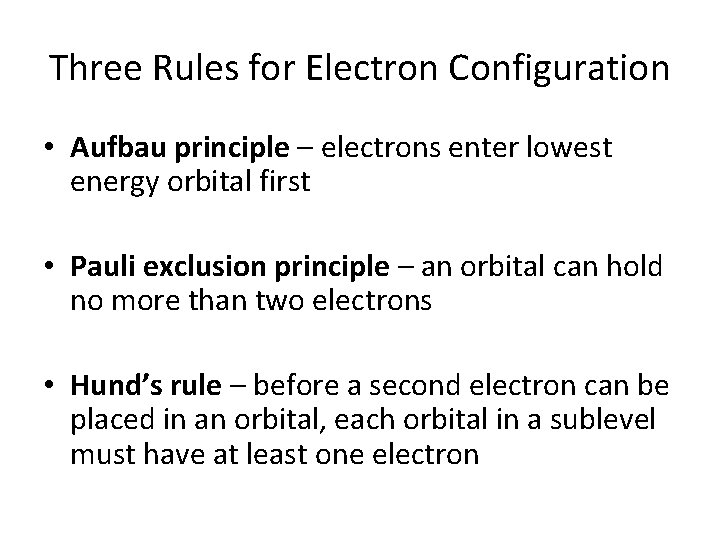
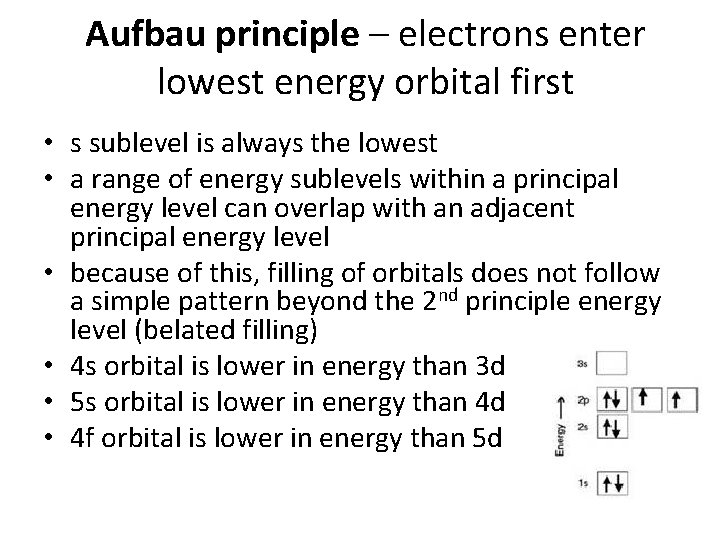
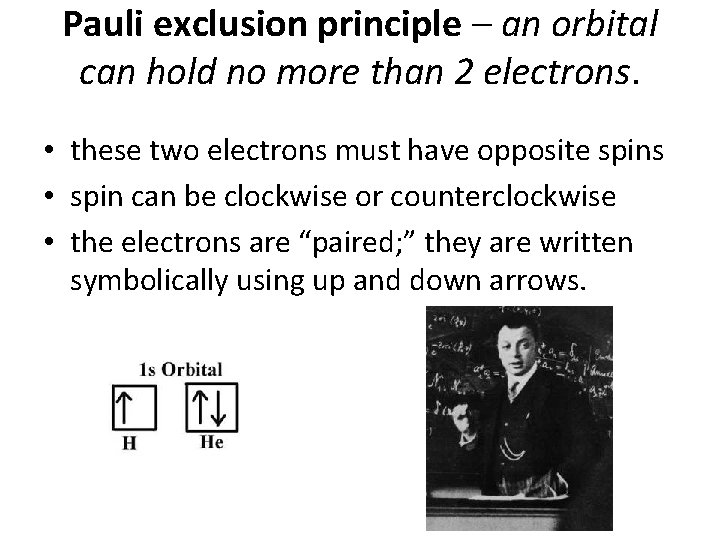
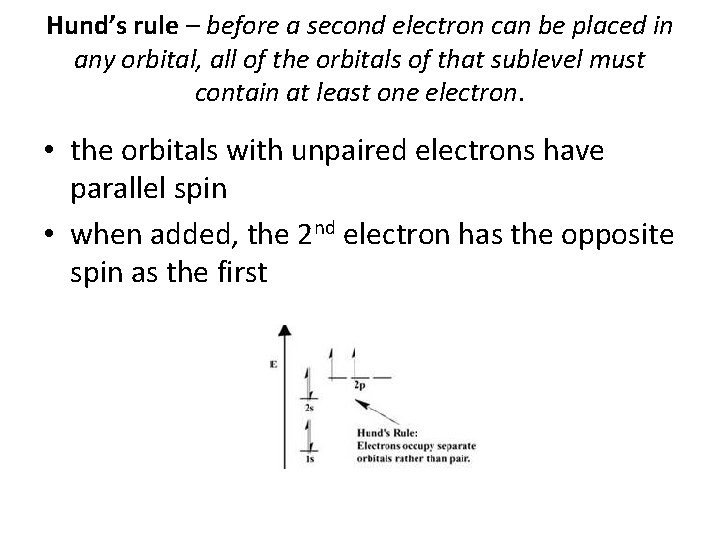
Three Rules for Electron Configuration • Aufbau principle – electrons enter lowest energy orbital first • Pauli exclusion principle – an orbital can hold no more than two electrons • Hund’s rule – before a second electron can be placed in an orbital, each orbital in a sublevel must have at least one electron

Putting electrons in their places. .

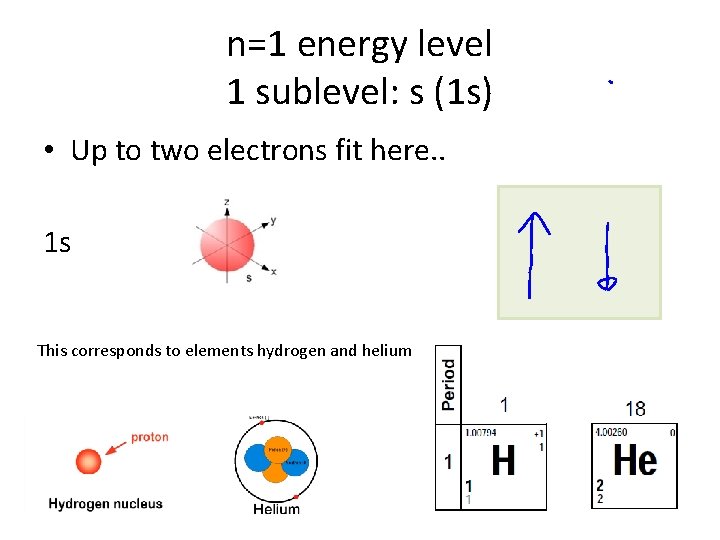
n=1 energy level 1 sublevel: s (1 s) • Up to two electrons fit here. . 1 s This corresponds to elements hydrogen and helium

n=2 energy level first sublevel: 2 s • This corresponds to elements Li and Be

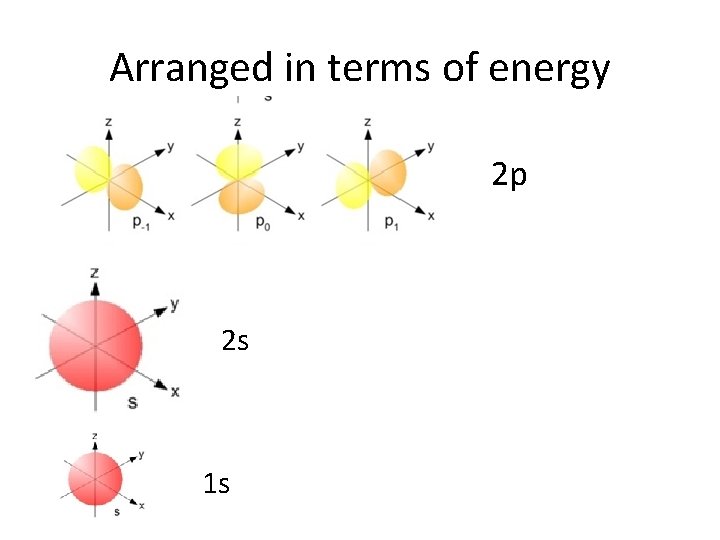
Arranged in terms of energy 2 s 1 s

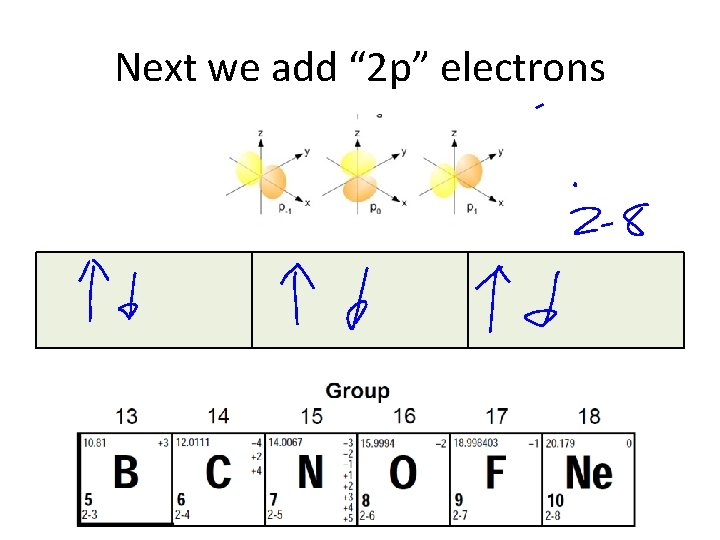
Next we add “ 2 p” electrons

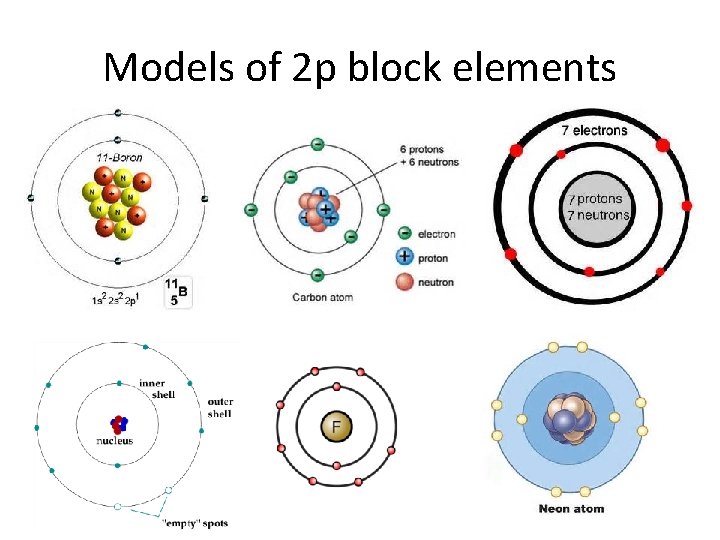
Models of 2 p block elements

Arranged in terms of energy 2 p 2 s 1 s

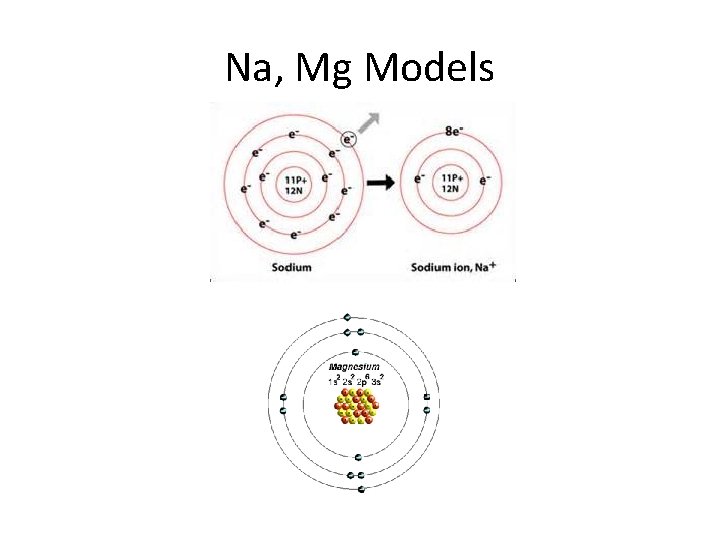
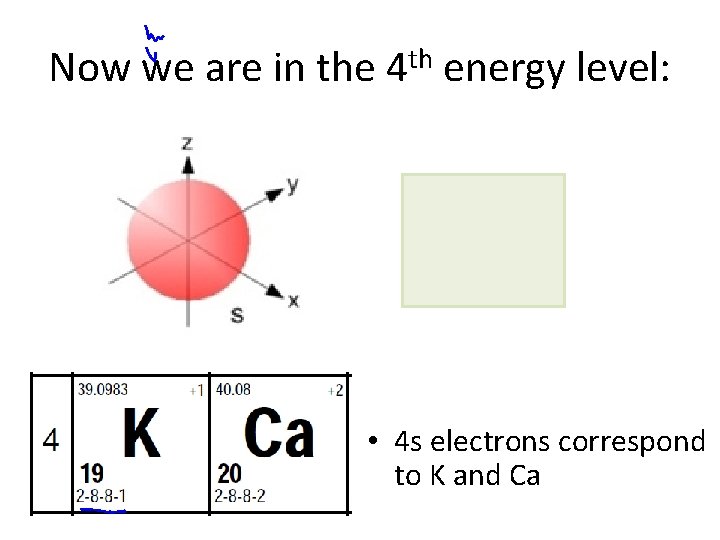
No we are in 3 rd energy level: • 3 s electrons correspond to Na and Mg

Na, Mg Models

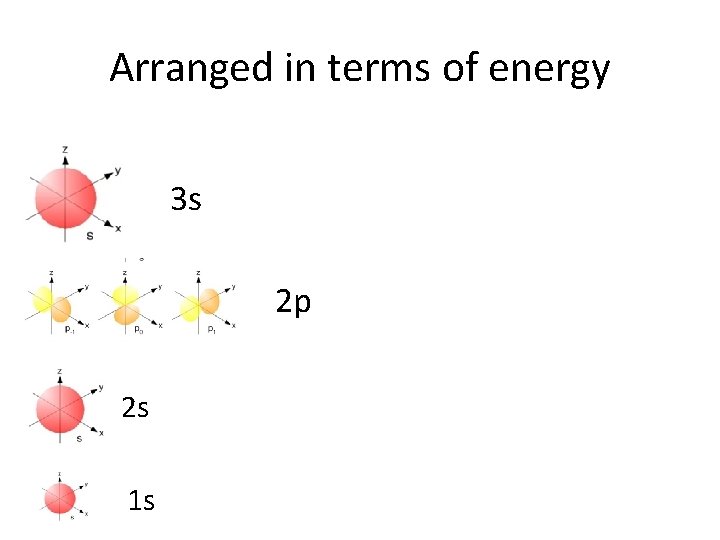
Arranged in terms of energy 3 s 2 p 2 s 1 s

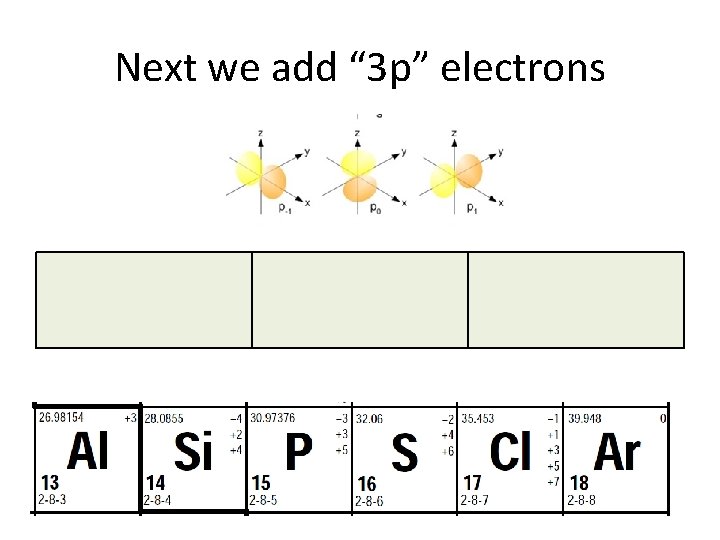
Next we add “ 3 p” electrons

Arranged in terms of energy 3 p 3 s 2 p 2 s 1 s

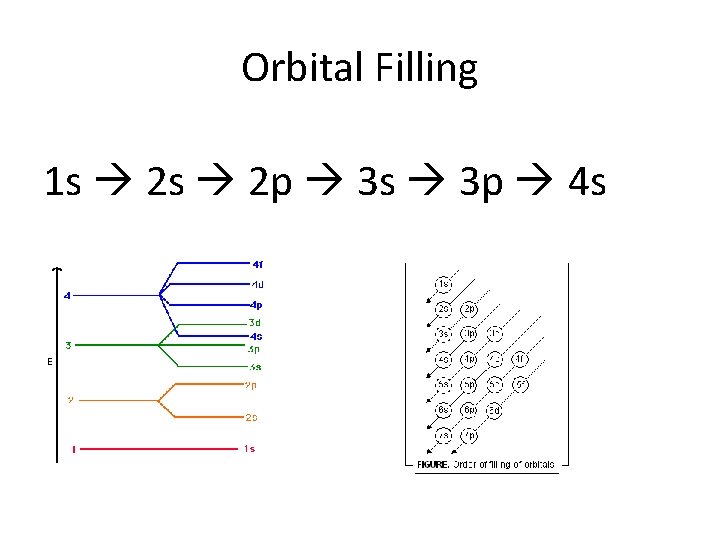
Overlapping Sublevels • As we move to higher energy levels, they get closer together in terms of overall energy. • s sublevels from a “higher” energy level often have lower energy d sublevels from lower levels • The 4 s sublevel is filled before the last sublevel of the 3 rd energy level (3 d)

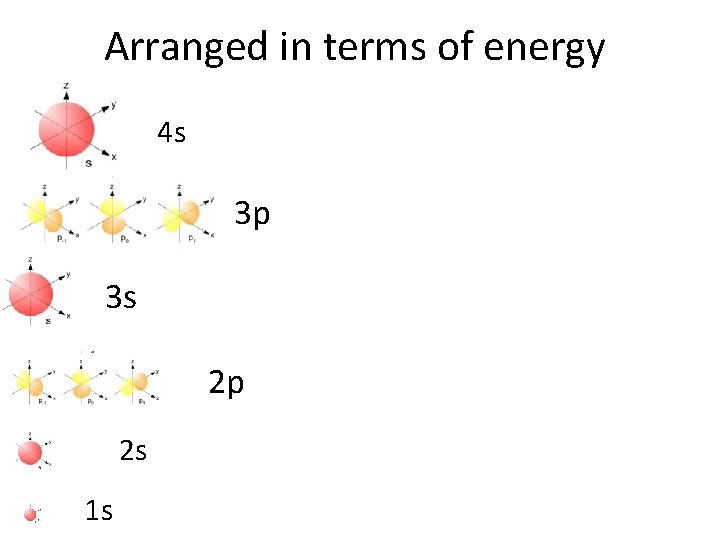
Now we are in the 4 th energy level: • 4 s electrons correspond to K and Ca

Arranged in terms of energy 4 s 3 p 3 s 2 p 2 s 1 s

Now (finally) the d electrons • 5 orbitals, 2 electrons each

Aufbau principle – electrons enter lowest energy orbital first • s sublevel is always the lowest • a range of energy sublevels within a principal energy level can overlap with an adjacent principal energy level • because of this, filling of orbitals does not follow a simple pattern beyond the 2 nd principle energy level (belated filling) • 4 s orbital is lower in energy than 3 d • 5 s orbital is lower in energy than 4 d • 4 f orbital is lower in energy than 5 d

Orbital Filling 1 s 2 p 3 s 3 p 4 s

Pauli exclusion principle – an orbital can hold no more than 2 electrons. • these two electrons must have opposite spins • spin can be clockwise or counterclockwise • the electrons are “paired; ” they are written symbolically using up and down arrows.

Hund’s rule – before a second electron can be placed in any orbital, all of the orbitals of that sublevel must contain at least one electron. • the orbitals with unpaired electrons have parallel spin • when added, the 2 nd electron has the opposite spin as the first

Lab 5 Probability of Finding an Electron • Where is a 1 s electron most likely to be found? • Materials (per group of two students) Sheet of paper, marking pen, compass

Procedure • Working with your partner, draw a target on the sheet of paper. The center represents the atom’s nucleus, which is very small in comparison to the rest of the atom. • The nucleus should be represented by a single dot in the center of the paper. • Draw concentric circles around the dot so that the radius of each circle is 2. 0 cm greater than that of the preceding circle.

• Tape the paper to the floor so it will not move. • Stand on the opposite side of the target from your partner. Holding the marker at shoulder height above the center of the target, take turns dropping the marker so that it leaves a dot when it hits the target. • Each dot represents the location of an electron at that moment.

• Continue dropping the marker until you each have 50 “electron” dots on your target. • Your partner should repeat the process for a total of 100 dots. • Count the number of dots in each ring and record that number in the data table below.

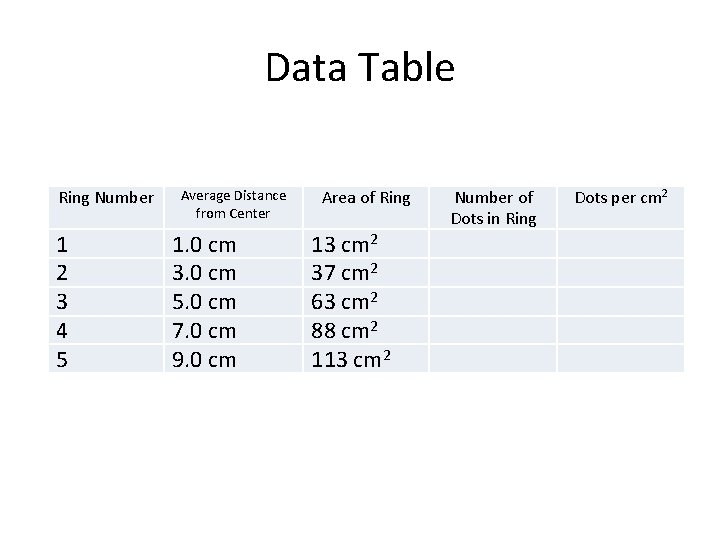
Data Table Ring Number 1 2 3 4 5 Average Distance from Center 1. 0 cm 3. 0 cm 5. 0 cm 7. 0 cm 9. 0 cm Area of Ring 13 cm 2 37 cm 2 63 cm 2 88 cm 2 113 cm 2 Number of Dots in Ring Dots per cm 2

Observations • Record the number of dots in each ring of the target and calculate the number of dots per square centimeter. • You take your experimental values in column 3, and divide them by the area in column 2! • What happens to the number of dots per unit area as the distance from the center increases?

Making a Graph • Make a graph of your observations by plotting the average distance from the center on the xaxis and the number of dots per cm 2 on the yaxis. • Be sure to put an appropriate title on your graph, and label each axis. • What does this graph represent? What would be a good title for the graph?

Discussion • Determine the probability of finding a dot in each of the rings in the target by dividing the number of dots in the ring by the total number of dots (100). • Multiplying this fraction by 100 will give you the percent probability. • Based on your graph, what is the distance with the highest probability of finding a dot? • How many dots are actually located at the distance of highest probability?

• Will there necessarily be an electron at the distance with the highest probability of finding an electron? Explain your answer. • In what ways is this model similar to the structure of an atom? In what ways is it different? • An atomic orbital is defined as the region within which an electron is found 90% of the time. On your target, draw a circle within which 90% of the dots are found. • What is the distance of this circle from the center? What is the area of this circle? Show your work.