1 Quantized Radiation Particle Theory of Light Dr












- Slides: 40

1 Quantized Radiation (Particle Theory of Light) Dr. Bill Pezzaglia Part I Updated: 2010 Apr 19

2 Quantum Mechanics A. Classical vs Quantum Theory B. Black Body Radiation C. Photoelectric Effect D. Atomic Physics

3 A. Classical vs Quantum Theory 1. Discussion in class. Essentially classical theory assumes: • Causality (causes must precede effects, i. e. can’t go backwards in time) • Determinism: classical theory assumes can measure quantities to infinite precision • Objectivity: classical theory assumes measurement does not affect the system • Two Models: particles (things) which can be localized vs waves (fields) which are non-local. Fields mediate the forces between particles Quantum theory upsets these ideas

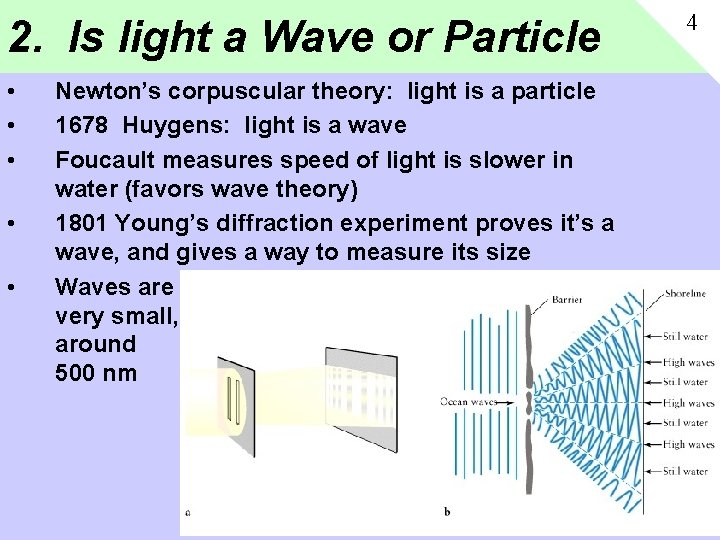
2. Is light a Wave or Particle • • • Newton’s corpuscular theory: light is a particle 1678 Huygens: light is a wave Foucault measures speed of light is slower in water (favors wave theory) 1801 Young’s diffraction experiment proves it’s a wave, and gives a way to measure its size Waves are very small, around 500 nm 4

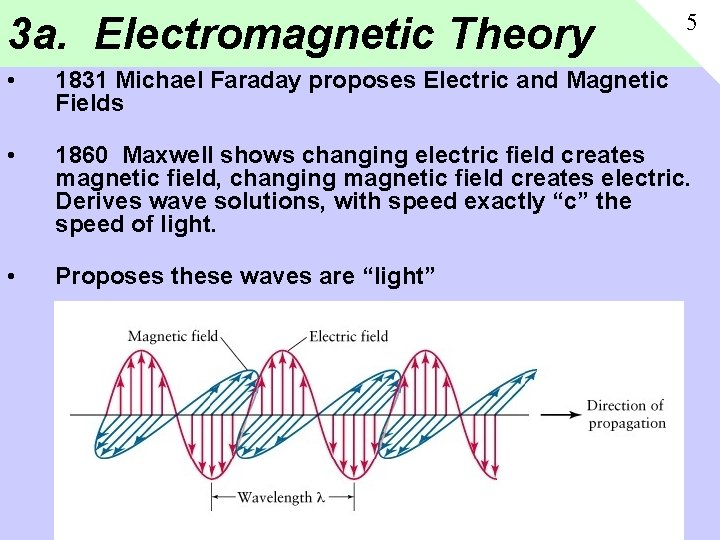
3 a. Electromagnetic Theory 5 • 1831 Michael Faraday proposes Electric and Magnetic Fields • 1860 Maxwell shows changing electric field creates magnetic field, changing magnetic field creates electric. Derives wave solutions, with speed exactly “c” the speed of light. • Proposes these waves are “light”

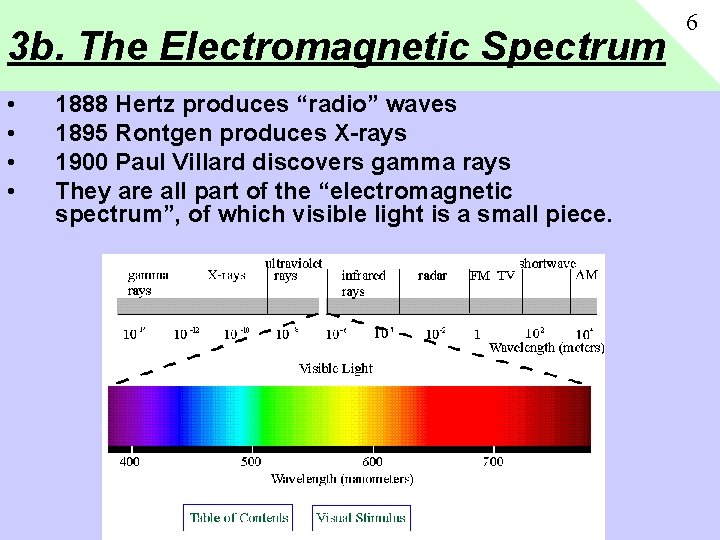
3 b. The Electromagnetic Spectrum • • 1888 Hertz produces “radio” waves 1895 Rontgen produces X-rays 1900 Paul Villard discovers gamma rays They are all part of the “electromagnetic spectrum”, of which visible light is a small piece. 6

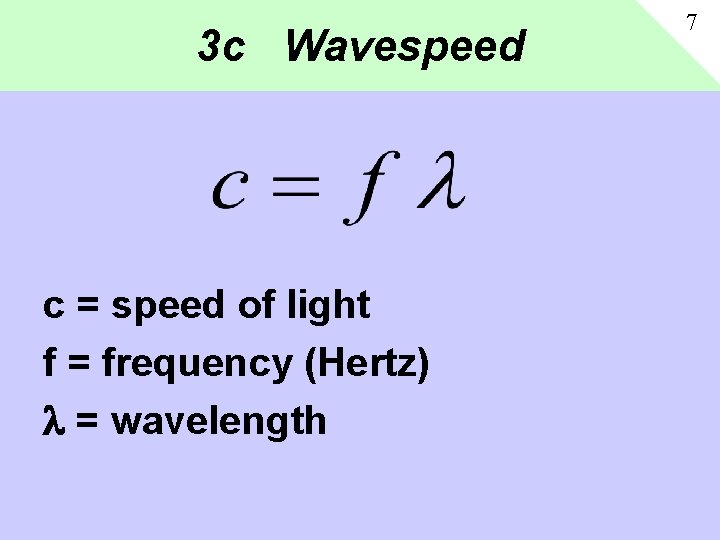
3 c Wavespeed c = speed of light f = frequency (Hertz) = wavelength 7

8 B. Black Body Radiation 1. Stephan Boltzmann Law 2. Wien’s Law 3. Black Body Radiation

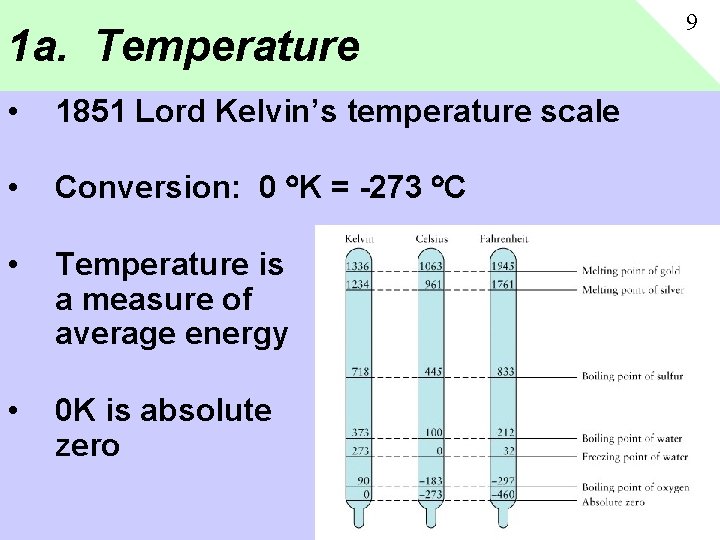
1 a. Temperature • 1851 Lord Kelvin’s temperature scale • Conversion: 0 K = -273 C • Temperature is a measure of average energy • 0 K is absolute zero 9

1 b. Josef Stefan’s Law 1879 • Experimentally shows total output of light of a hot dense (black) body is proportional to 4 th power of the temperature (in Kelvin) • • Power (watts)=A T 4 • =5. 67 x 10 -8 Watts/(m 2 -K 4) A=surface area • 1884 Ludwig Boltzmann (former student of Stefan) derives formula from thermodynamics. • I was a guest speaker (Sept 2005) at the Josef Stefan Institute in Slovenia. 10

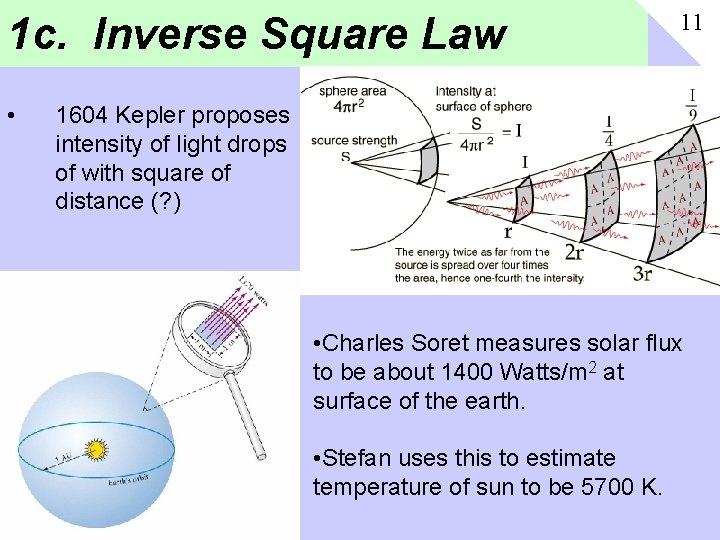
1 c. Inverse Square Law • 11 1604 Kepler proposes intensity of light drops of with square of distance (? ) • Charles Soret measures solar flux to be about 1400 Watts/m 2 at surface of the earth. • Stefan uses this to estimate temperature of sun to be 5700 K.

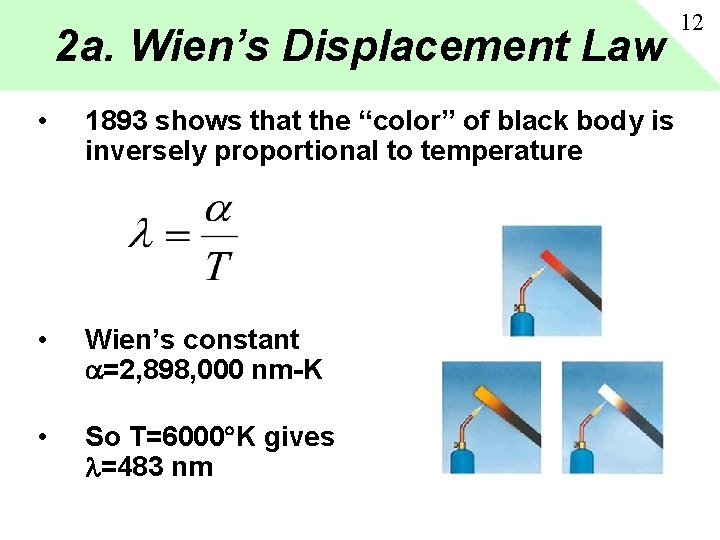
2 a. Wien’s Displacement Law • 1893 shows that the “color” of black body is inversely proportional to temperature • Wien’s constant =2, 898, 000 nm-K • So T=6000 K gives =483 nm 12

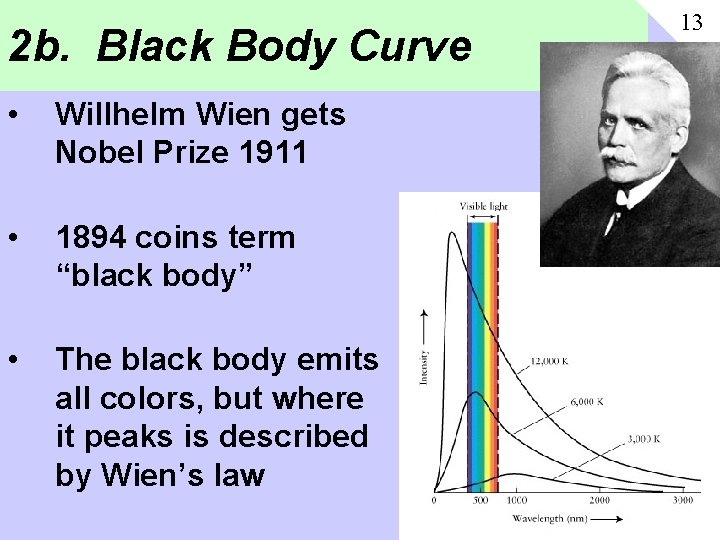
2 b. Black Body Curve • Willhelm Wien gets Nobel Prize 1911 • 1894 coins term “black body” • The black body emits all colors, but where it peaks is described by Wien’s law 13

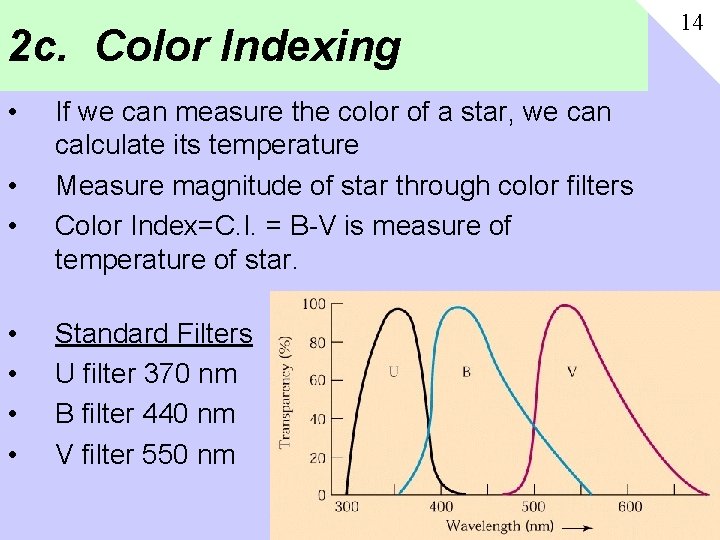
2 c. Color Indexing • • If we can measure the color of a star, we can calculate its temperature Measure magnitude of star through color filters Color Index=C. I. = B-V is measure of temperature of star. Standard Filters U filter 370 nm B filter 440 nm V filter 550 nm 14

3 a. Black Body Theory • Maxwell: hot atoms vibrate, acting like small antennas, radiating electromagnetic waves • Wien tries to give theory to explain shape of curve, but it fails in IR • Rayleigh (1900) & Jeans (1905) have another theory, but it fails in UV, blowing up to infinite energy (the “ultraviolet catastrophe”). 15

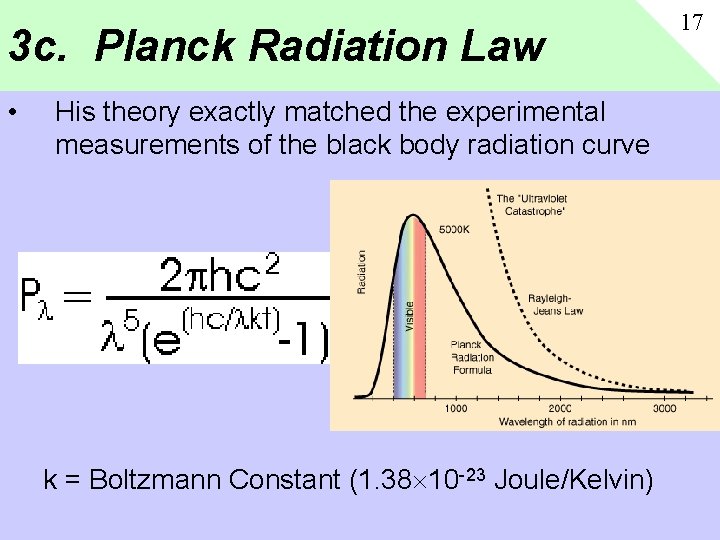
3 b. Max Planck’s Theory • 1900 Max Planck ad-hoc proposes that vibrations are “quantized”, i. e. come in steps of n=1, 2, 3, rather than continuous. • Energy: E=nhf n=integer quantum number f=frequency of oscillation h is “Planck’s Constant h=6. 626 x 10 -34 Joule-Sec 16

3 c. Planck Radiation Law • His theory exactly matched the experimental measurements of the black body radiation curve k = Boltzmann Constant (1. 38 10 -23 Joule/Kelvin) 17

18 C. Photoelectric Effect 1. Phenomena Discovered 2. The Experiment 3. Einstein’s Photon Theory

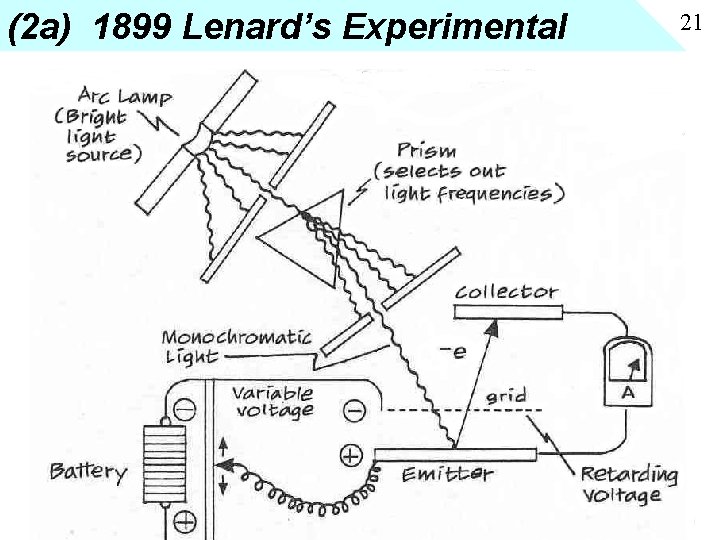
19 1. Discovery of Effect • Hertz 1887 found discharge of a spark gap was greater when illuminated by light from another spark gap. Glass inserted between stopped effect (note glass absorbs uv light) • 1888 Hallwachs discovers that negatively charge electroscope will discharge when UV light is shined on it, but not with visible light. No effect for positive charged electroscope.

20 1 b. Work Function There is a certain amount of energy required to free an electron from the metal (between 2 and 3 volts) • • • Cesium 1. 9 volts Calcium 2. 7 volts Magnesium 3. 6 volts Use energy of light to add energy and “bump off” electrons.

(2 a) 1899 Lenard’s Experimental 21

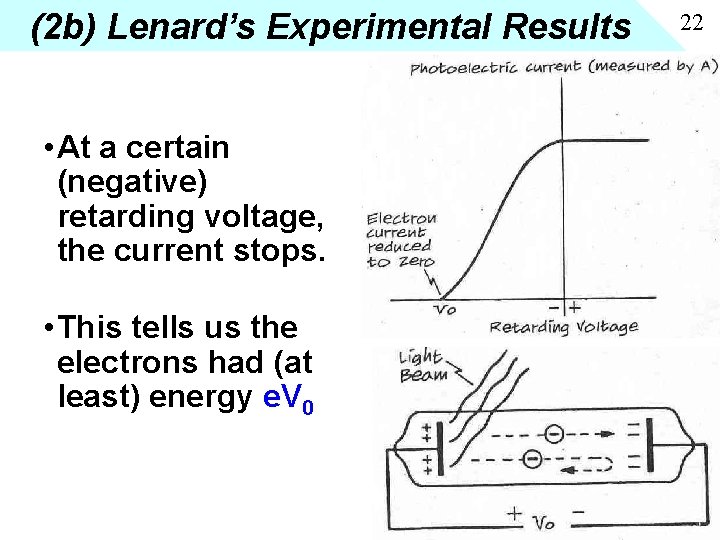
(2 b) Lenard’s Experimental Results • At a certain (negative) retarding voltage, the current stops. • This tells us the electrons had (at least) energy e. V 0 22

(2 c) Findings • More intense light, more current, but electrons do not have more energy (stopping voltage unchanged) • Higher frequency light, electrons have higher energy (bigger stopping voltage) • Below a certain threshold frequency of light, there is NO current, regardless of the intensity • Even when intensity is so low that it should take an hour to give enough energy to overcome the work function of the metal, if photon is above threshold, the effect is instantaneous. 23

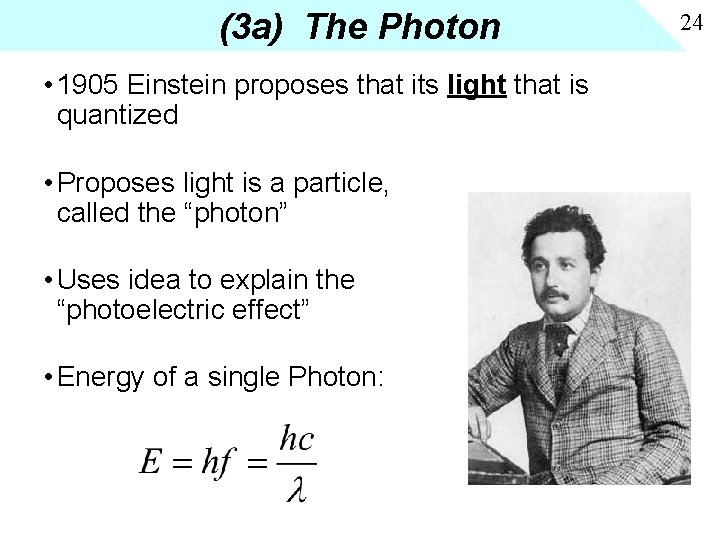
(3 a) The Photon • 1905 Einstein proposes that its light that is quantized • Proposes light is a particle, called the “photon” • Uses idea to explain the “photoelectric effect” • Energy of a single Photon: 24

3 b. Einstein • 1905 proposed Photon concept, with quantized energy E=hf • Emission is caused by ONE photon bumping off ONE electron. Photon frequency must be such that energy is above work function • Millikan makes precise measurement of stopping voltage vs frequency and gets straight line (different for each metal), with slope (h/e) and intercept gives work function. 25

26 D. Atomic Physics 1. Discrete Spectra 2. Kirchhoff’s Laws 3. Model of Atom

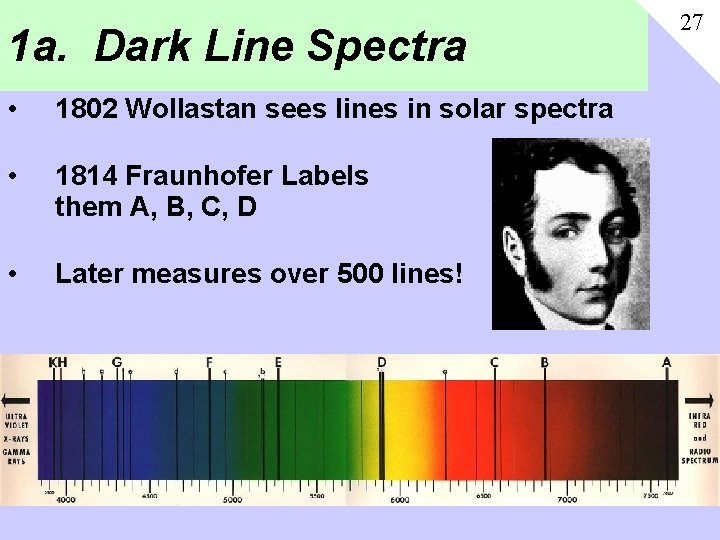
1 a. Dark Line Spectra • 1802 Wollastan sees lines in solar spectra • 1814 Fraunhofer Labels them A, B, C, D • Later measures over 500 lines! 27

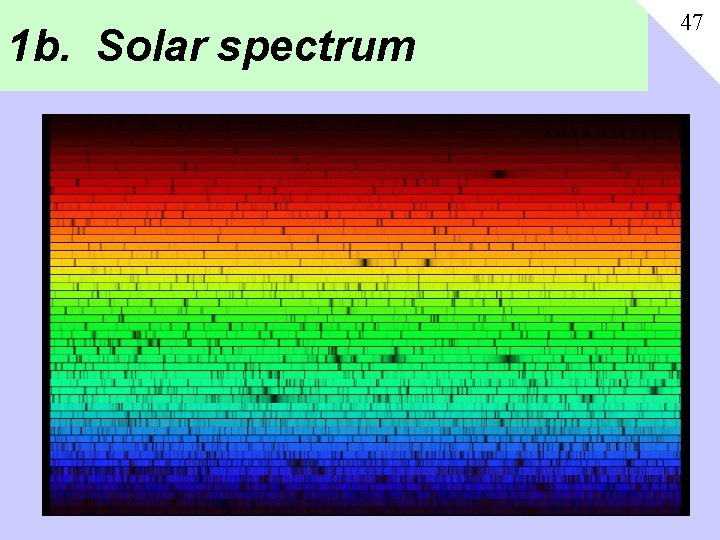
1 b. Solar spectrum 47

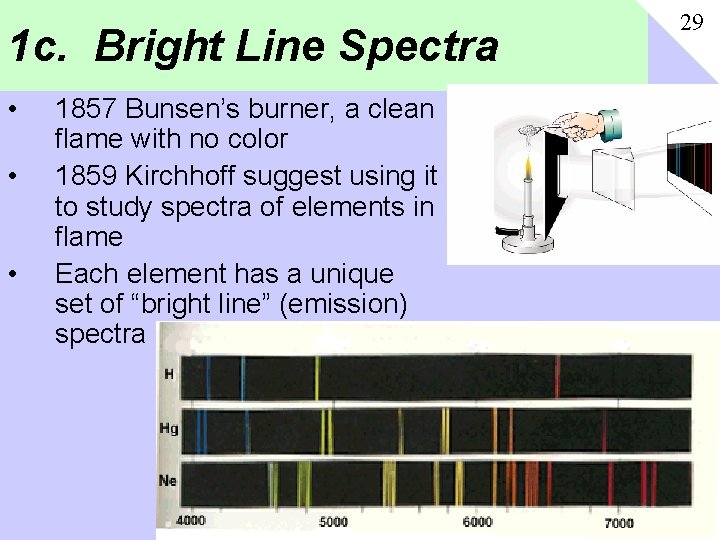
1 c. Bright Line Spectra • • • 1857 Bunsen’s burner, a clean flame with no color 1859 Kirchhoff suggest using it to study spectra of elements in flame Each element has a unique set of “bright line” (emission) spectra 29

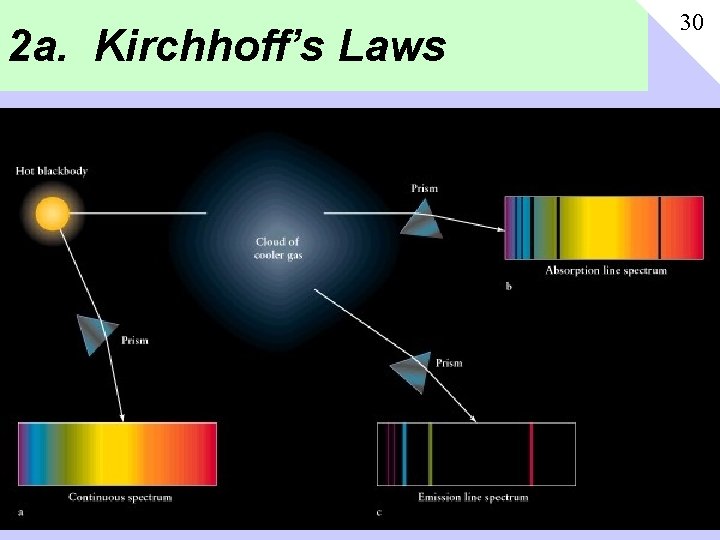
2 a. Kirchhoff’s Laws 30

2 b. Gustav R Kirchhoff (1860) His three laws: 1. A hot dense body will emit a continuous spectrum 2. A hot transparent gas will emit emission line spectrum 3. A cool transparent gas in front of a source of continuous spectrum will produce absorption spectra. 31

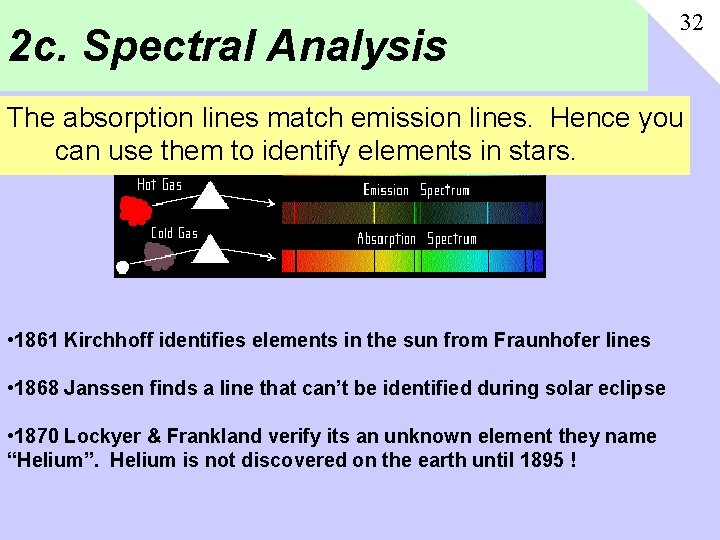
2 c. Spectral Analysis 32 The absorption lines match emission lines. Hence you can use them to identify elements in stars. • 1861 Kirchhoff identifies elements in the sun from Fraunhofer lines • 1868 Janssen finds a line that can’t be identified during solar eclipse • 1870 Lockyer & Frankland verify its an unknown element they name “Helium”. Helium is not discovered on the earth until 1895 !

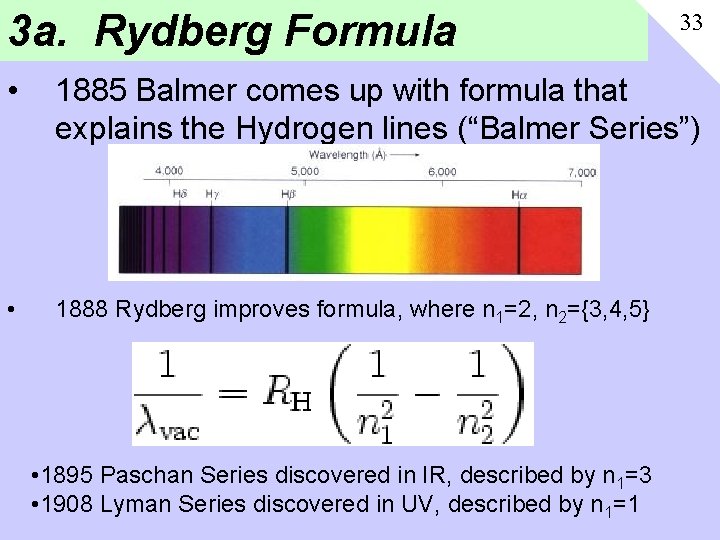
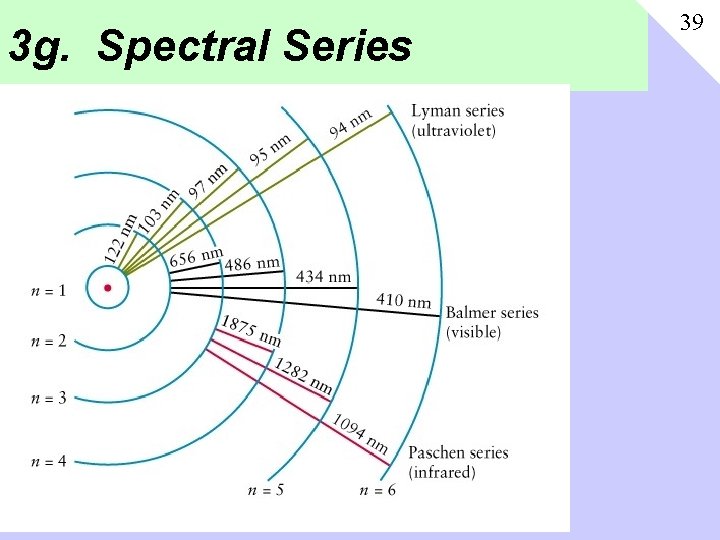
3 a. Rydberg Formula 33 • 1885 Balmer comes up with formula that explains the Hydrogen lines (“Balmer Series”) • 1888 Rydberg improves formula, where n 1=2, n 2={3, 4, 5} • 1895 Paschan Series discovered in IR, described by n 1=3 • 1908 Lyman Series discovered in UV, described by n 1=1

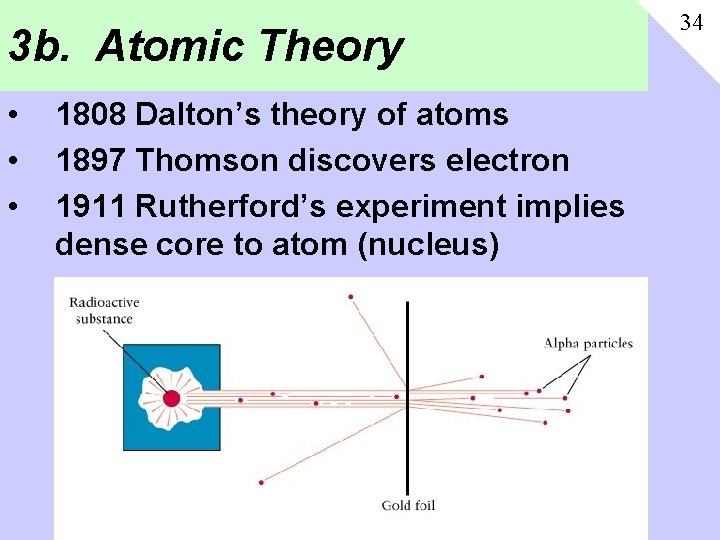
3 b. Atomic Theory • • • 1808 Dalton’s theory of atoms 1897 Thomson discovers electron 1911 Rutherford’s experiment implies dense core to atom (nucleus) 34

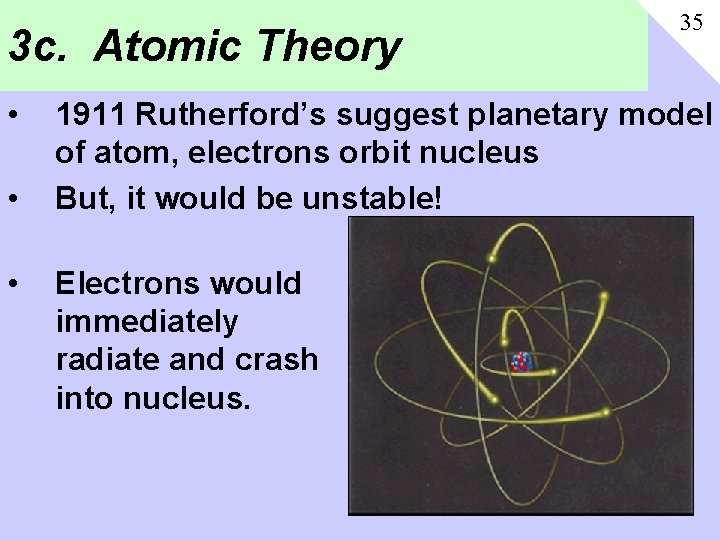
3 c. Atomic Theory • • • 35 1911 Rutherford’s suggest planetary model of atom, electrons orbit nucleus But, it would be unstable! Electrons would immediately radiate and crash into nucleus.

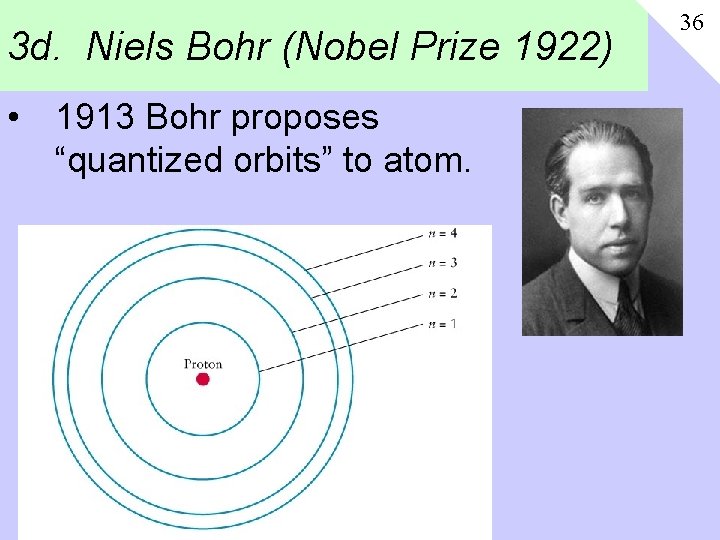
3 d. Niels Bohr (Nobel Prize 1922) • 1913 Bohr proposes “quantized orbits” to atom. 36

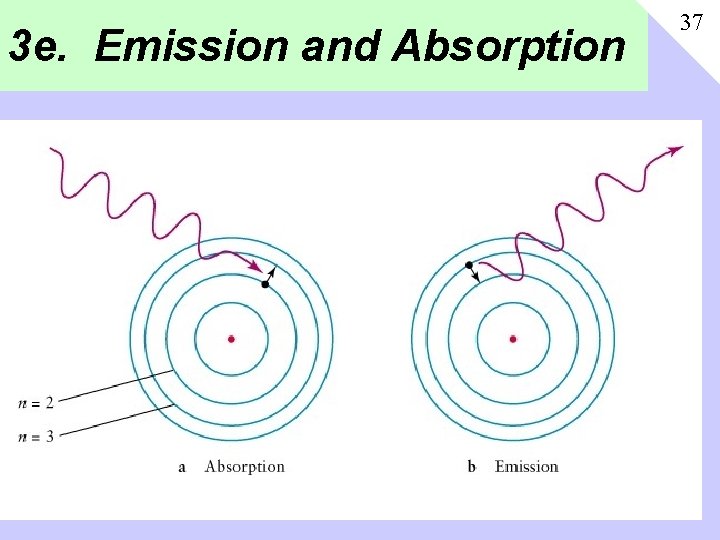
3 e. Emission and Absorption 37

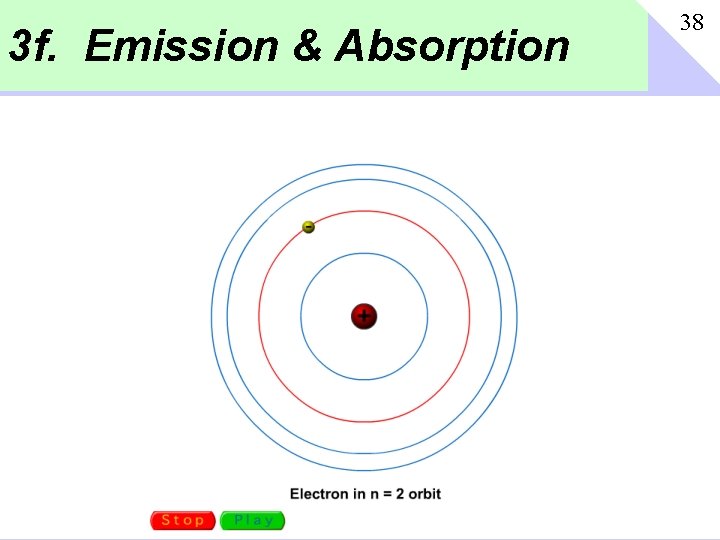
3 f. Emission & Absorption 38

3 g. Spectral Series 39

References • Mc. Evoy & Zarate, “Introducing Quantum Theory” (Totem Books, 1996) 40
 Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Light and quantized energy
Light and quantized energy Section 1 light and quantized energy
Section 1 light and quantized energy Particle theory of light
Particle theory of light Isaac newton theory of light
Isaac newton theory of light Quantized inertia
Quantized inertia Light light light chapter 23
Light light light chapter 23 Into the light chapter 22
Into the light chapter 22 Light light light chapter 22
Light light light chapter 22 What is tachyon
What is tachyon Light is a particle evidence
Light is a particle evidence Photon
Photon Wave speed equation
Wave speed equation Light behaves primarily as a particle when it
Light behaves primarily as a particle when it The particle model describes light as
The particle model describes light as Particle model of light
Particle model of light Metastable state
Metastable state Transverse wave vs longitudinal wave
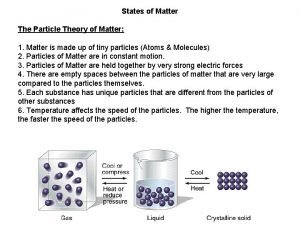
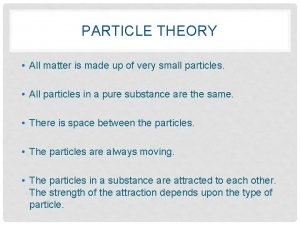
Transverse wave vs longitudinal wave Particle theory of matter
Particle theory of matter Particle theory of matter examples
Particle theory of matter examples Particle theory of matter
Particle theory of matter Conduction particle theory
Conduction particle theory Particle theory of matter grade 7
Particle theory of matter grade 7 5 particle theory of matter
5 particle theory of matter Particle theory of matter
Particle theory of matter Www.youtube.com
Www.youtube.com Particle theory of matter
Particle theory of matter Particle theory of matter grade 9
Particle theory of matter grade 9 Particle theory freezing
Particle theory freezing Solid to liquid endothermic or exothermic
Solid to liquid endothermic or exothermic Put out the light and then put out the light
Put out the light and then put out the light Membrane-bound organelles
Membrane-bound organelles The bouncing off of light.
The bouncing off of light. Block light materials
Block light materials Color theory 101
Color theory 101 Maxwell's theory of light
Maxwell's theory of light Additive colour theory
Additive colour theory Brightness and contrast
Brightness and contrast Riley scattering
Riley scattering Mie plot
Mie plot