The Particle of Light A particle model of



- Slides: 14

The Particle of Light • A particle model of light is necessary to describe phenomena observed in modern physics, for example, the interaction between light and atoms. PHYS 140 Light as a Particle 1

The Photoelectric Effect • • Many physicists’ work contributed to the discovery of the photoelectric effect What is it? • The ability of light to dislodge electrons from a metallic surface • The electrons can be detected and the resulting signals amplified • Lots of applications in visual imaging PHYS 140 Light as a Particle 2

Questions • • • How many electrons are ejected in a given time? How does this number depend of wavelength or intensity? How energetic are the ejected electrons? Upon what does the electron energy depend? Are electrons ejected instantly or is there a time delay? PHYS 140 Light as a Particle 3

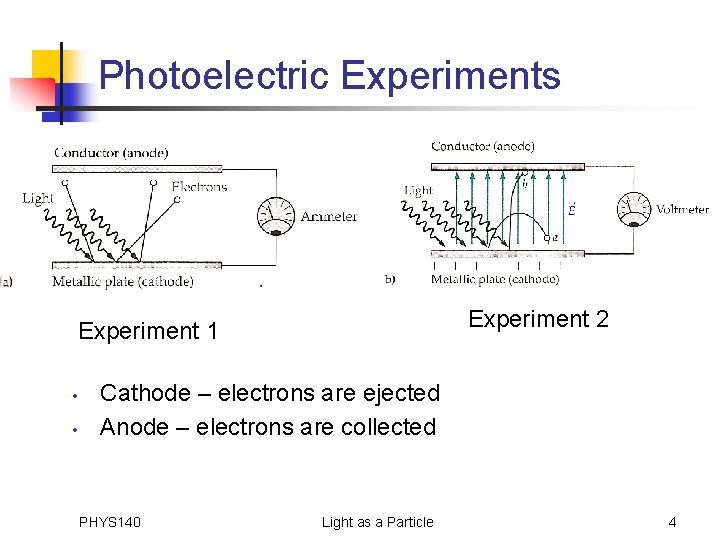
Photoelectric Experiments Experiment 2 Experiment 1 • • Cathode – electrons are ejected Anode – electrons are collected PHYS 140 Light as a Particle 4

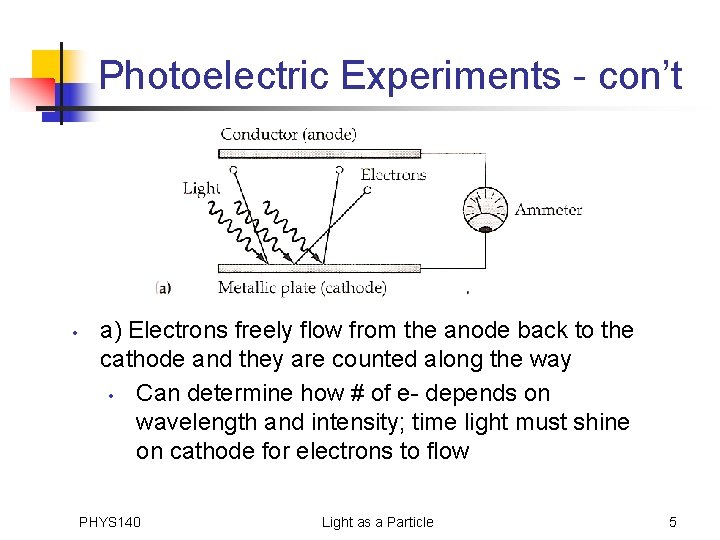
Photoelectric Experiments - con’t • a) Electrons freely flow from the anode back to the cathode and they are counted along the way • Can determine how # of e- depends on wavelength and intensity; time light must shine on cathode for electrons to flow PHYS 140 Light as a Particle 5

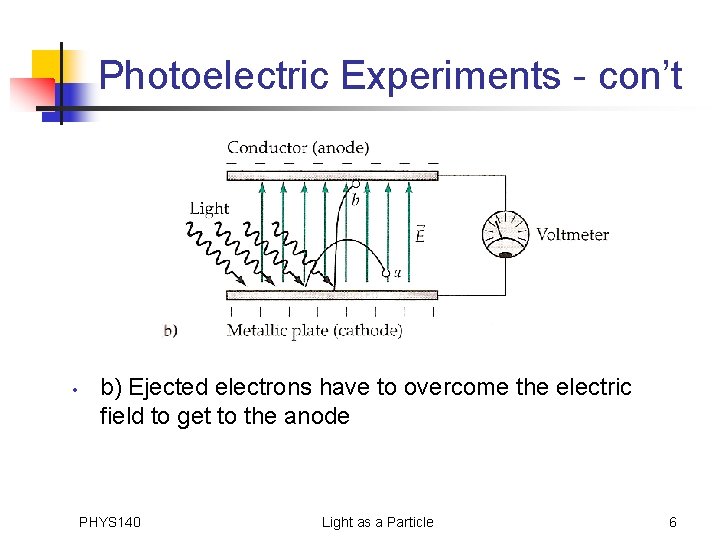
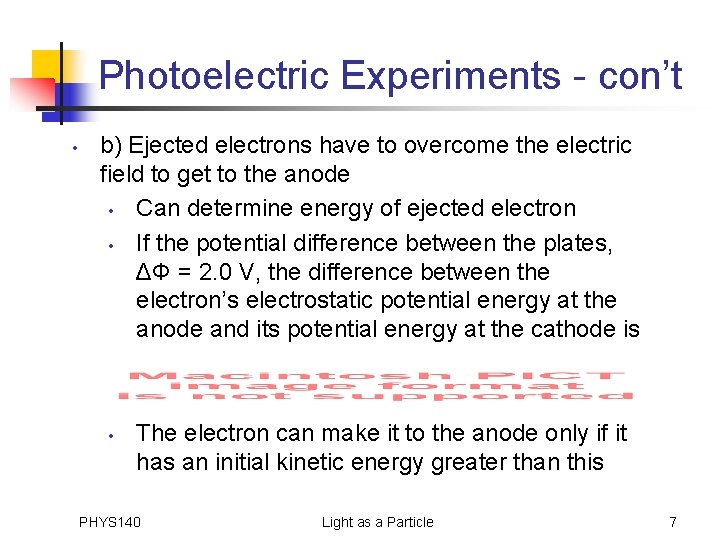
Photoelectric Experiments - con’t • b) Ejected electrons have to overcome the electric field to get to the anode PHYS 140 Light as a Particle 6

Photoelectric Experiments - con’t • b) Ejected electrons have to overcome the electric field to get to the anode • Can determine energy of ejected electron • If the potential difference between the plates, ΔΦ = 2. 0 V, the difference between the electron’s electrostatic potential energy at the anode and its potential energy at the cathode is • The electron can make it to the anode only if it has an initial kinetic energy greater than this PHYS 140 Light as a Particle 7

Wave Model Predictions • • The rate at which electrons are ejected from a metal is proportional to the intensity of the incident light. Lower intensity light rays should have a delay before electrons are ejected The rate may depend on frequency (wavelength) of light The maximum kinetic energy of the electrons is likely to increase with increasing intensity PHYS 140 Light as a Particle 8

Experiments Provide the Following Results • • • At high intensities and fixed frequencies, the # of ejected electrons is proportional to intensity Electrons are ejected instantly, regardless of intensity level For constant intensity, the # of electrons decreases with increasing frequency If the frequency is below a certain level, no electrons are ejected, regardless of intensity level Above the cutoff frequency, the electrons’ maximum kinetic energy is proportional to the frequency of light PHYS 140 Light as a Particle ✔ ✔ ✔ 9

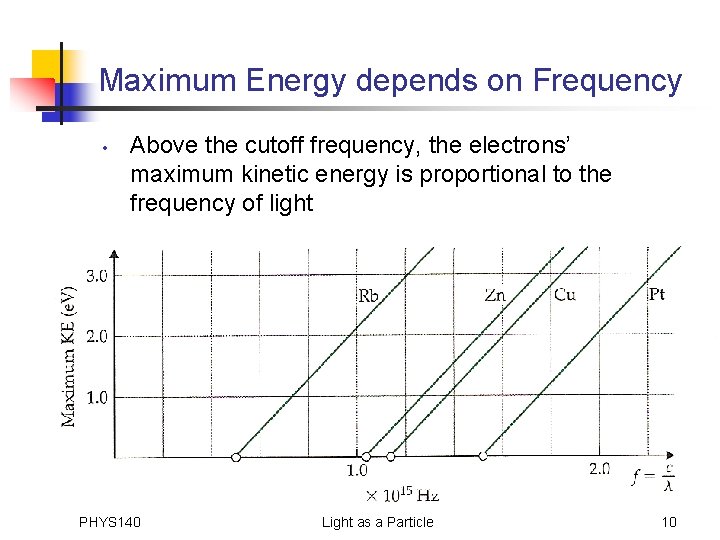
Maximum Energy depends on Frequency • Above the cutoff frequency, the electrons’ maximum kinetic energy is proportional to the frequency of light PHYS 140 Light as a Particle 10

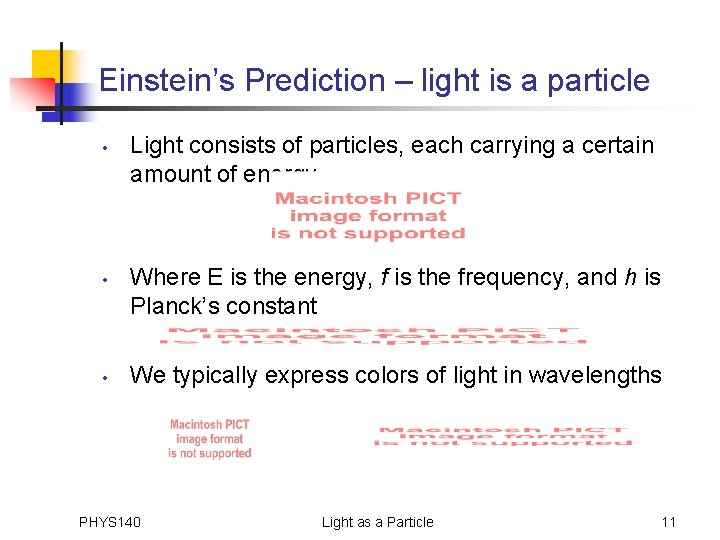
Einstein’s Prediction – light is a particle • • • Light consists of particles, each carrying a certain amount of energy Where E is the energy, f is the frequency, and h is Planck’s constant We typically express colors of light in wavelengths PHYS 140 Light as a Particle 11

Einstein’s Prediction - con’t • • Einstein also predicted that each electron ejected from the metal was a result of a collision with a single photon Where K is the kinetic energy of the electron and W is the work function for the metal • PHYS 140 The work function is the energy required to liberate the electron from the metal Light as a Particle 12

Einstein’s Prediction - con’t • Einstein’s model explains the experimental results so neatly, why was there resistance in the science community? • • This model is completely inconsistent with the wave nature of light. Neither model, wave or particle, adequately explains light by itself PHYS 140 Light as a Particle 13

Practice • Interactive activity – photoelectric effect for different metals • • http: //phet. colorado. edu/simulations/sims. php? sim =Photoelectric_Effect Group Problems • PHYS 140 Q 3 B. 5, Q 3 S. 4 Light as a Particle 14
 Light light light chapter 23
Light light light chapter 23 Light light light chapter 22
Light light light chapter 22 Light light light chapter 22
Light light light chapter 22 Draw an example of how light travels as a wave.
Draw an example of how light travels as a wave. The particle model describes light as
The particle model describes light as Particle model of light
Particle model of light Tachyons faster than light
Tachyons faster than light Light is a particle evidence
Light is a particle evidence Photon
Photon Particle theory of light
Particle theory of light Light behaves primarily as a particle when it
Light behaves primarily as a particle when it Proposed the particle theory of light
Proposed the particle theory of light Othello put out the light
Othello put out the light Leucoplast double membrane
Leucoplast double membrane The bouncing off of light.
The bouncing off of light.