Zerovalent iron ZVI removal of arsenic in a













- Slides: 23

Zero-valent iron (ZVI) removal of † arsenic in a spouted vessel J. M. Calo (JMCalo@brown. edu) J. Kirchner, L. Madhavan, and E. J. Bain Brown University School of Engineering Providence, RI 02912, USA Arsenic - Health and Remediation Applications NIEHS Webinar, April 15, 2013 † Project 5, Brown University Superfund Research Program, NIEHS 1

Overview What is a spouted bed? Spouted bed applications to heavy metals removal Our recent related work with arsenic removal Arsenic ZVI in a hybrid spouted bed/filter system - Experimental - Batch experiment results - Continuous flow system results - Results of arsenic ZVI in a hybrid spouted bed/filter - Summary - Continuing ZVI work Conclusions 2

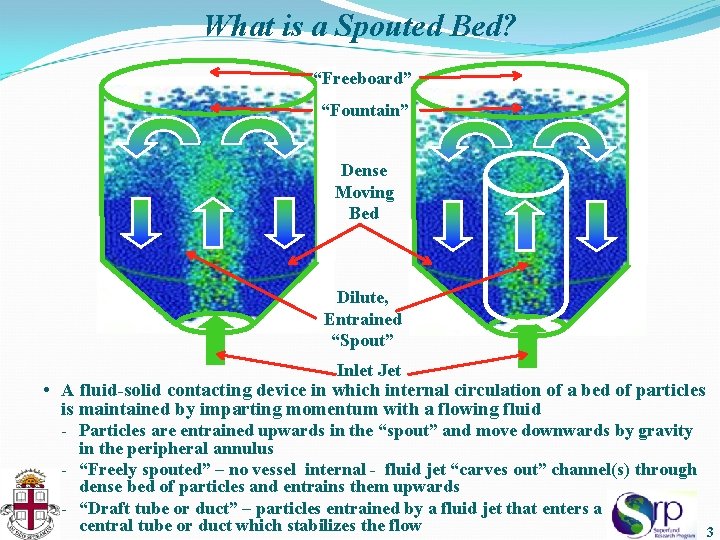
What is a Spouted Bed? “Freeboard” “Fountain” Dense Moving Bed Dilute, Entrained “Spout” Inlet Jet • A fluid-solid contacting device in which internal circulation of a bed of particles is maintained by imparting momentum with a flowing fluid - Particles are entrained upwards in the “spout” and move downwards by gravity in the peripheral annulus - “Freely spouted” – no vessel internal - fluid jet “carves out” channel(s) through dense bed of particles and entrains them upwards - “Draft tube or duct” – particles entrained by a fluid jet that enters a central tube or duct which stabilizes the flow 3

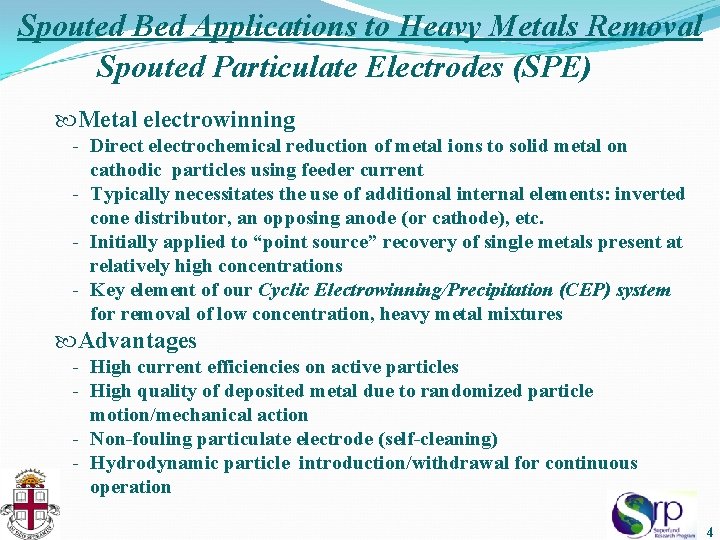
Spouted Bed Applications to Heavy Metals Removal Spouted Particulate Electrodes (SPE) Metal electrowinning - Direct electrochemical reduction of metal ions to solid metal on cathodic particles using feeder current - Typically necessitates the use of additional internal elements: inverted cone distributor, an opposing anode (or cathode), etc. - Initially applied to “point source” recovery of single metals present at relatively high concentrations - Key element of our Cyclic Electrowinning/Precipitation (CEP) system for removal of low concentration, heavy metal mixtures Advantages - High current efficiencies on active particles - High quality of deposited metal due to randomized particle motion/mechanical action - Non-fouling particulate electrode (self-cleaning) - Hydrodynamic particle introduction/withdrawal for continuous operation 4

Spouted Particulate Electrode (SPE) 5

Spouted Particulate Electrode (SPE) 6

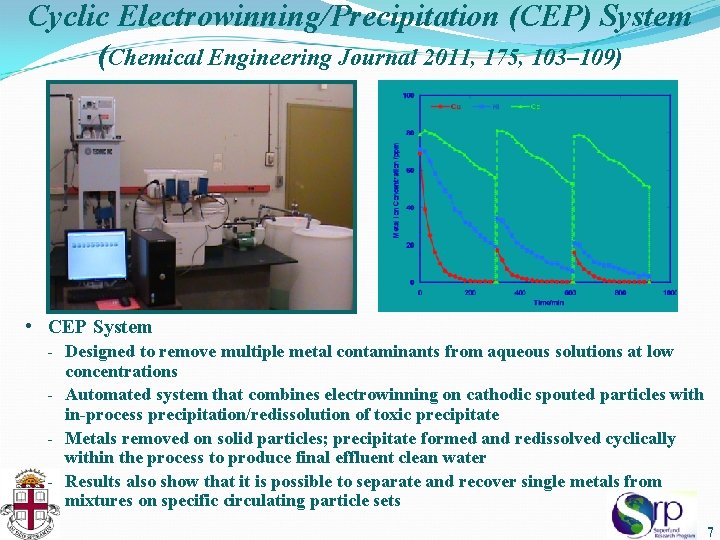
Cyclic Electrowinning/Precipitation (CEP) System (Chemical Engineering Journal 2011, 175, 103– 109) • CEP System - Designed to remove multiple metal contaminants from aqueous solutions at low concentrations - Automated system that combines electrowinning on cathodic spouted particles with in-process precipitation/redissolution of toxic precipitate - Metals removed on solid particles; precipitate formed and redissolved cyclically within the process to produce final effluent clean water - Results also show that it is possible to separate and recover single metals from mixtures on specific circulating particle sets 7

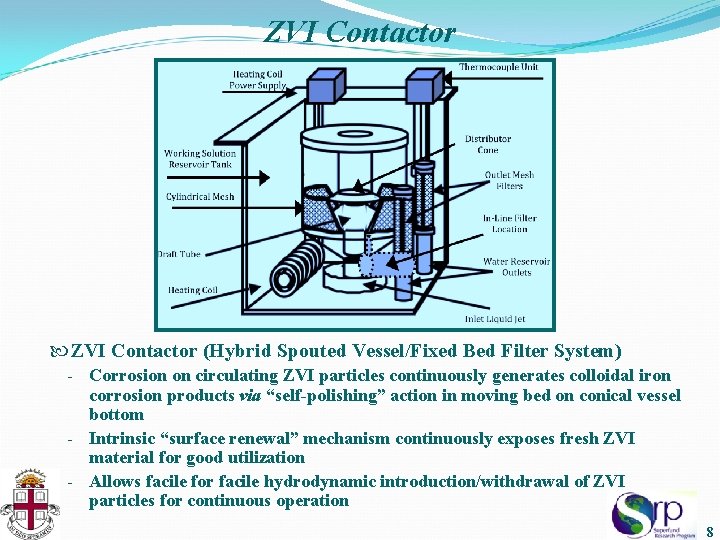
ZVI Contactor (Hybrid Spouted Vessel/Fixed Bed Filter System) Corrosion on circulating ZVI particles continuously generates colloidal iron corrosion products via “self-polishing” action in moving bed on conical vessel bottom - Intrinsic “surface renewal” mechanism continuously exposes fresh ZVI material for good utilization - Allows facile for facile hydrodynamic introduction/withdrawal of ZVI particles for continuous operation - 8

Our Recent Related Work With Arsenic Electrosorption/Electrodesorption on Carbons (Energy & Fuels, 2010, 24(6), 3415– 3421) Electrosorption - adsorption under the influence of an electric field - Effective for removal of heavy metal anions (As, Cr) - Increases adsorption rate and sorbent capacity - Reversing the field desorbs As quantitatively (electrodesorption) provides for adsorbent regeneration Electrochemical Cell Co-contaminant metals (e. g. , Cr, Fe, etc. ) can also enhance As removal via various mechanisms - electro-precipitation, complexation, etc. , on carbon adsorbent active sites 9 9

Cyclic Arsenic Removal on a Carbon Electrode (Electrochimica Acta, 2009, 54, 3996– 4004) • Arsenic redox on porous carbon electrode deposited on a quartz crystal microbalance (continuous electrode mass measurement) • Observed increase in arsenic uptake with each cycle - Due to creation of additional active carbon sites for As adsorption/reduction produced by carbon electrogasification over redox cycle - Cyclic adsorption/desorption could be used to increase adsorbent capacity and effectiveness (during reduction), as well as providing for adsorbent regeneration (during oxidation) 10 10

Zero-Valent Iron (ZVI) Removal of Arsenic (Chem. Eng. J. 2012, 189 – 190, 237– 243) • Removal of aqueous arsenic species with zero-valent iron (ZVI) is a well-known, abiotic process that occurs on ZVI materials as they corrode in water - Arsenic species removed irreversibly from solution via a mechanism involving adsorption, co-precipitation, and surface complexation with ZVI-generated iron oxides, hydroxides, and oxide-hydroxide corrosion products - Some typical problems include: + On colloidal corrosion products - relatively low rates due to low encounter frequencies between arsenic species and colloidal particles in solution + On bulk ZVI - limited ZVI material utilization due to diffusion barrier formation 11

Experimental Iron particles - Grade 100, 1/8” (3. 175 mm) diameter, carbon steel spheres (98. 2 - 99. 2 wt % iron) - Salem Specialty Ball Company, Inc. Cylindrical (5 in. long, 2. 5 in. O. D. ) 20 µm (FOS-398 304) stainless steel, in-line filter (ISC Sales, Inc. ) installed in solution reservoir of spouted vessel system Arsenic solutions prepared as 1000 mg/L standard solutions of either As(III) or As(V) As 2 O 3 standard prepared with sodium hydroxide, and then subsequently acidified with nitric acid (Trace. SELECT) + Resultant As(III) 30 mg/L stock solution was buffered at p. H 4 with potassium hydrogen phthalate (KHP) and sodium hydroxide (1. 492 g/L KHP, 8 mg/L Na. OH) - As(V) 30 mg/L stock solution, including 1. 5 g/L of Na. Cl, was prepared from the 1000 mg/L As(V) standard solution initially made up from 99. 9% (arsenic basis) arsenic (V) pentoxide (Alfa Aesar) - Total arsenic concentrations determined with Perkin Elmer 4100 ZL Zeeman Effect Graphite Furnace Atomic Absorption Spectrometer (GFAAS) 12

Batch Experiment Results • To simulate ZVI particle behavior in packed column (immobilized) and spouted vessel (agitated) - 100 ppb As(III) in p. H 4 buffered solution - Immobilized: spheres in PP mesh bags suspended in stirred solution - Agitated: spheres in sample tubes mounted on mechanical rotator • Results - Immobilized: no arsenic removal initially; no visible corrosion products on particles until ~ 198. 5 h; ultimately ~10% arsenic removal - Agitated: arsenic removal ~45% in 24 h; corrosion products visible after ~ 1 h • Conclusions - Apparent arsenic removal kinetics orders of magnitude more rapid for the agitated samples - Results consistent with “surface renewal” mechanism hypothesis 13

Continuous Flow System Experiment Results • Packed column and spouted vessel contactors - Packed column – ¾” diameter tube - Each charged with same amount (270. 1 g) of ZVI spheres • Results - Particles in spouted vessel exhibit about an order of magnitude greater mass loss than in comparable fixed bed experiments; i. e. , 24. 3 vs. 2. 5 g over a 10 h run (p. H 4) Conclusions - Packed column promotes the formation of a heavy coating of corrosion products on ZVI sphere surfaces; eventually occluding liquid flow in the column - Spouted vessel promotes the formation of colloidal corrosion products in the bulk liquid phase due to the self-polishing surface renewal mechanism - Results consistent with “surface renewal” mechanism hypothesis 14

Arsenic ZVI in a Hybrid Spouted Bed/Filter System • Arsenic removal by ZVI in a spouted vessel - “Rust” (iron oxides and oxyhydroxides) is generated on circulating ZVI particles - “Self-polishing” action of the particles in the dense moving bed on the conical vessel bottom, continuously removes surface rust, exposing fresh ZVI to corrosion - “Surface renewal” mechanism creates a continuous source of active “rust” generation • Multiple reservoirs of corrosion products in the spouted vessel system: - On surfaces of circulating primary ZVI particles - As colloidal corrosion products in the bulk liquid phase - As colloidal corrosion products adsorbed onto vessel surfaces - The observed arsenic removal behavior is dependent on the relative importance of the various corrosion product “reservoirs” 15

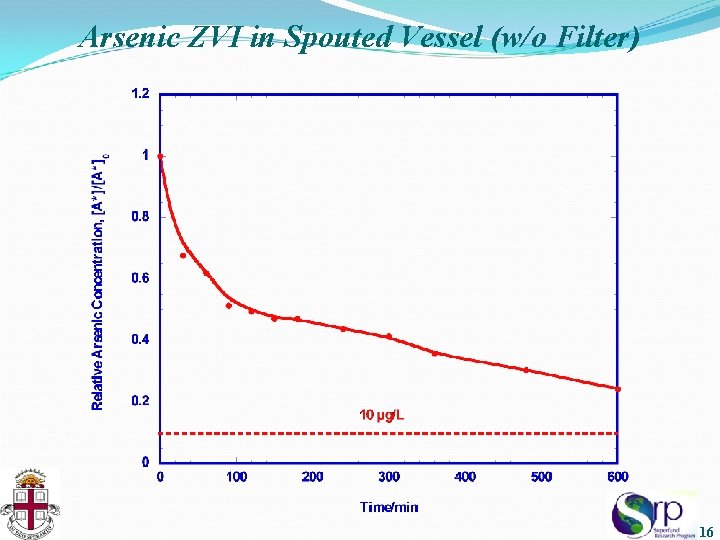
Arsenic ZVI in Spouted Vessel (w/o Filter) 16

Arsenic ZVI in Spouted Vessel With Filter) 17

Comparison of Arsenic Removal Rates 18

Summary of Results • Arsenic was removed, but only very slowly, with just the ZVI particles circulating in the spouted vessel - From 100 µg/L, the As concentration still exceeded 10 µg/L (ppb) after 10 h - A numerical bimodal fit to the data indicated approximately half the arsenic was complexed by iron corrosion products adsorbed onto vessel surfaces (faster mode), and the other half by colloidal corrosion particles circulating in the bulk liquid solution (slower mode) • Operation with the filter installed on one of the two reservoir drain lines dramatically accelerated arsenic removal to below the USEPA MCL in less than one hour - Kinetic behavior of arsenic concentration controlled by increased “encounter frequency” of arsenic species with colloidal corrosion particles concentrated in the filter 19

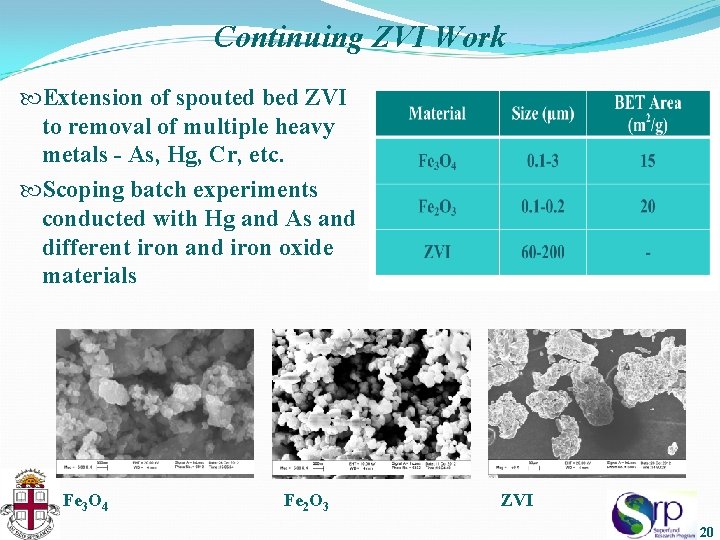
Continuing ZVI Work Extension of spouted bed ZVI to removal of multiple heavy metals - As, Hg, Cr, etc. Scoping batch experiments conducted with Hg and As and different iron and iron oxide materials Fe 3 O 4 Fe 2 O 3 ZVI 20

Continuing ZVI Work – Some Results Arsenic Removal - Rates of As(III) and As(V) removal considerably greater on iron oxides than ZVI - Removal of As is more p. H dependent for ZVI than the oxides – viz. , coupled to corrosion rate Hg(II) Removal − ZVI material significantly outperformed Fe 2 O 3 and Fe 3 O 4 in 500 ppm Na. Cl and Na 2 SO 4 solutions + Equilibrium uptake: 52 and 26 mg Hg/g, respectively - Effective rates considerably greater on ZVI as well - Large differences in removal capacity of ZVI, Fe 3 O 4 and Fe 2 O 3 indicate that Hg reduction-precipitation plays a major role in the mechanism 21

Conclusions The hybrid spouted vessel/fixed bed filter system was demonstrated to be very effective for arsenic removal from aqueous solutions at low concentrations (e. g. , to meet drinking water standards) - Effective treatment times can be reduced by one or two orders of magnitude, proportional to the increase in the effective water treatment rate - This performance can be improved upon, since the current system, as tested, has not been optimized with respect to the filter properties or the geometry/operating conditions of the spouted vessel system The novel hybrid nature of the system lies in its dualfunction character, whereby colloidal material is continuously generated in the moving bed on the spouted vessel bottom, and arsenic removal/complexation occurs in the fixed bed filter 22

Conclusions - This method of generating colloidal corrosion products maximizes utilization of ZVI material in comparison to other methods, such as with fixed bulk iron sources - Operation of the hybrid system is simple and cost effective + Requires little attention - periodic backwashing of the filter to remove collected colloidal material + With the current system under the operating conditions tested, the filter reaches capacity after about 28 h on stream This is sufficient to remove essentially all the arsenic at an initial concentration of 100 µg/L to less than detectable limits (<<10µg/L) from about 800 L or 213 gal of water, or 7. 8 gal/h, utilizing only about 7. 5% of the initial charge of ZVI material in a relatively small device - ZVI particles can be introduced hydrodynamically as required - ZVI particles do not have to be continuously recirculated – periodic or pulsed circulation - The system can be readily scaled-up (e. g. , with greater filter and liquid capacities, etc. ) 23
 Formula for arsenic pentabromide
Formula for arsenic pentabromide Verbal irony in arsenic and old lace
Verbal irony in arsenic and old lace Sono arsenic filter
Sono arsenic filter Ground state of arsenic
Ground state of arsenic The atomic radius in periodic table
The atomic radius in periodic table Ground state electron configuration of arsenic
Ground state electron configuration of arsenic Collapsibility test for plastic containers
Collapsibility test for plastic containers Shorthand electron configuration for arsenic
Shorthand electron configuration for arsenic Lewis structure for arsenic
Lewis structure for arsenic Hans gross
Hans gross Lewis dot diagram for rubidium
Lewis dot diagram for rubidium Rb3p ionic or covalent
Rb3p ionic or covalent Type 2 binary ionic compounds
Type 2 binary ionic compounds Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship Zvi wiener
Zvi wiener Corporate strategy examples
Corporate strategy examples Zvi aronson
Zvi aronson Dr zvi margaliot
Dr zvi margaliot Andrea reger
Andrea reger Zvi wiener
Zvi wiener Zvi roth
Zvi roth Zvi margaliot
Zvi margaliot Zvi kedem
Zvi kedem