Technologies for Arsenic Removal Tom Sorg U S

























- Slides: 41

Technologies for Arsenic Removal Tom Sorg U. S. Environmental Protection Agency

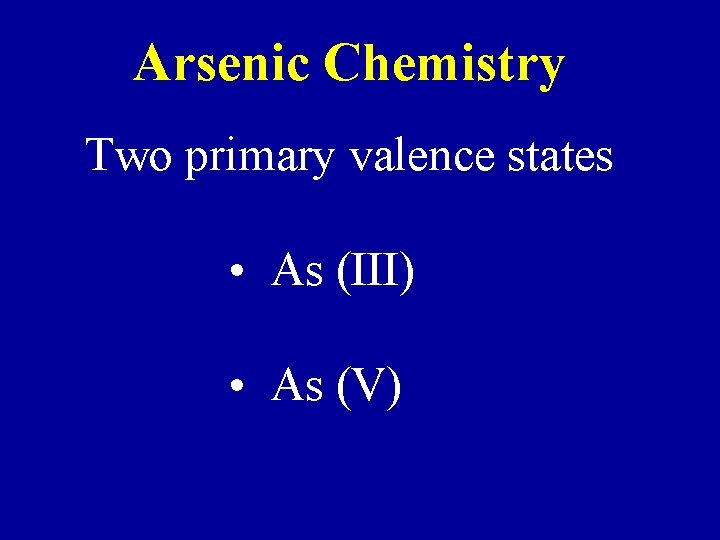
Arsenic Chemistry Two primary valence states • As (III) • As (V)

Arsenic III H 3 As. O 3 0 H 2 As. O 3 -1 -2 HAs. O 3

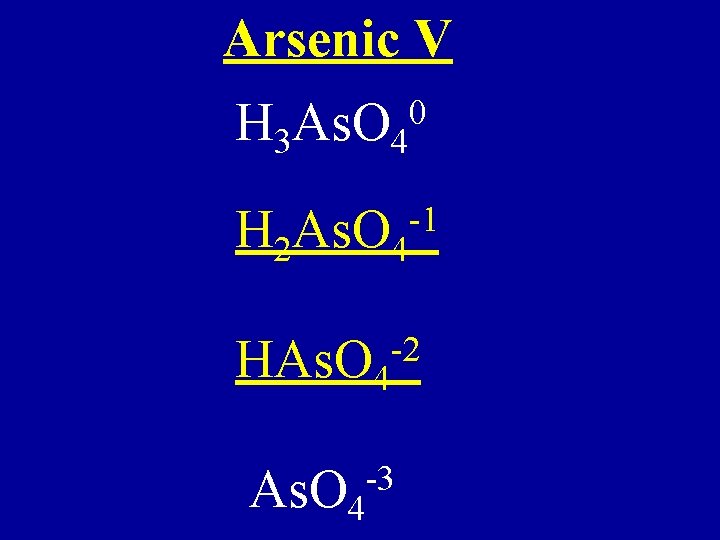
Arsenic V H 3 As. O 4 0 H 2 As. O 4 -1 -2 HAs. O 4 -3 As. O 4

Why is arsenic form important? Final Answer! As V more effectively removed by ALL technologies

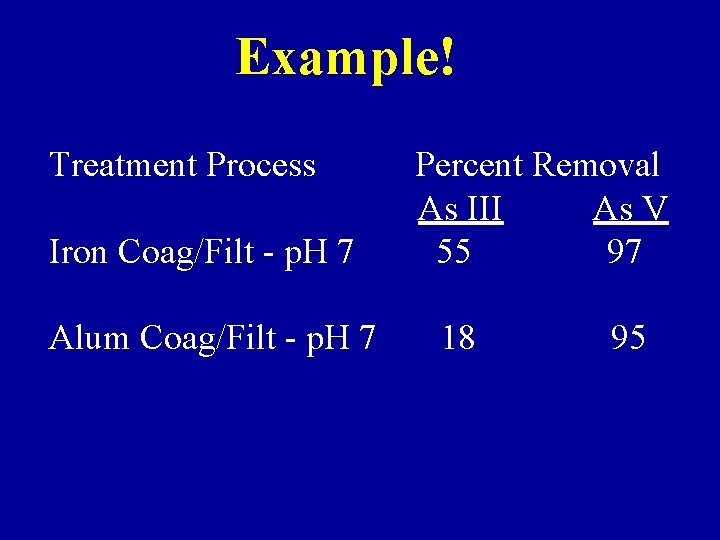
Example! Treatment Process Iron Coag/Filt - p. H 7 Alum Coag/Filt - p. H 7 Percent Removal As III As V 55 97 18 95

Example! Ion exchange treatment As III - 0 percent removal As V - 98+ percent removal

Arsenic Occurrence Surface waters predominantly As(V) Ground waters generally As(III), but not always

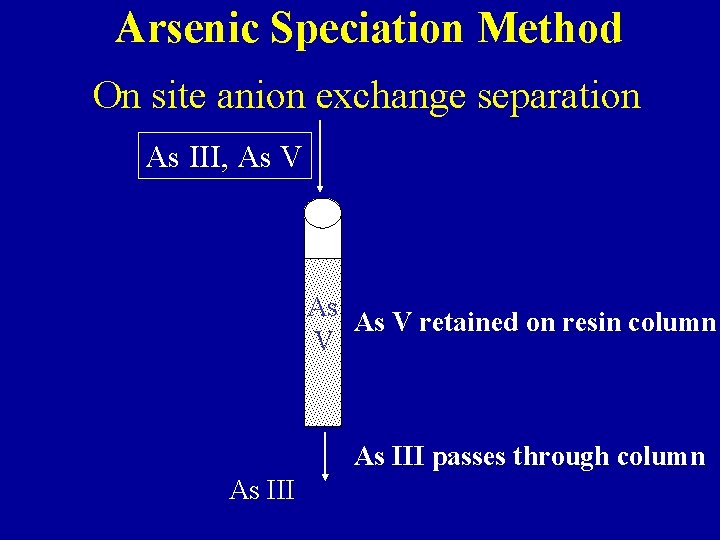
Arsenic Speciation Method On site anion exchange separation As III, As V As As V retained on resin column V As III passes through column As III

Arsenic Speciation - Anion separation of As. III/As. V

Good News! As III easily oxidized to As V by several oxidants

As III Oxidation Study Dr. Dennis Clifford Univ. of Houston i Oxidants Studied 1. Free Chlorine 2. Chloramine 3. Ozone 4. Chlorine Dioxide 5. UV Radiation 6. Potassium Permanganate 7. Oxidizing Media

Arsenic Removal Processes • Precipitative processes • Adsorption processes • Ion Exchange process • Iron Removal processes • Membrane processes • POU/POE devices

Arsenic Removal Processes Emerging processes Iron coagulation with microfiltration Iron based adsorption media

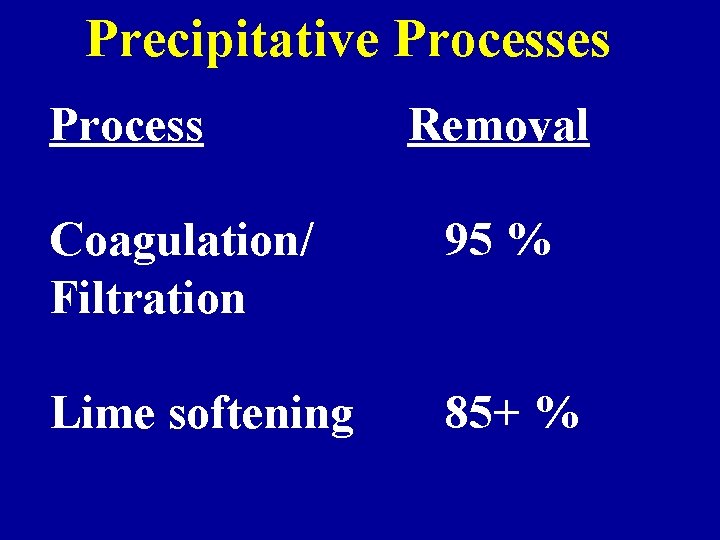
Precipitative Processes Process Removal Coagulation/ Filtration 95 % Lime softening 85+ %

Adsorption Processes Removal Activated Alumina 90+ % Iron Media 90+ %

Ion Exchange 95 + % removal

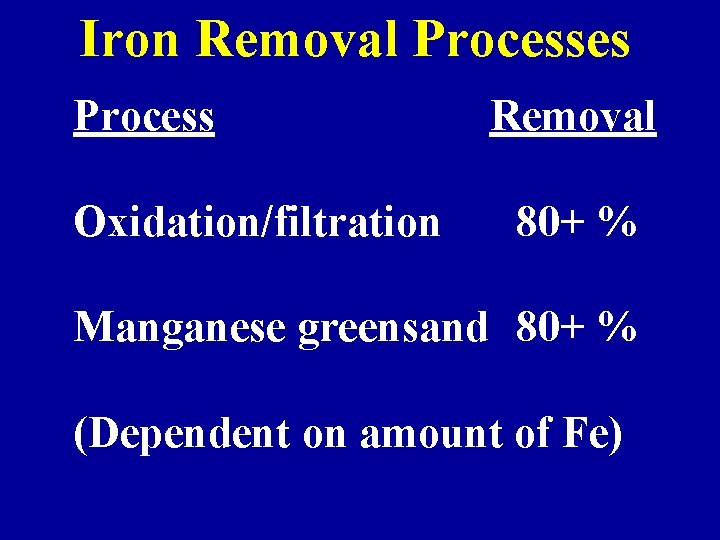
Iron Removal Processes Process Oxidation/filtration Removal 80+ % Manganese greensand 80+ % (Dependent on amount of Fe)

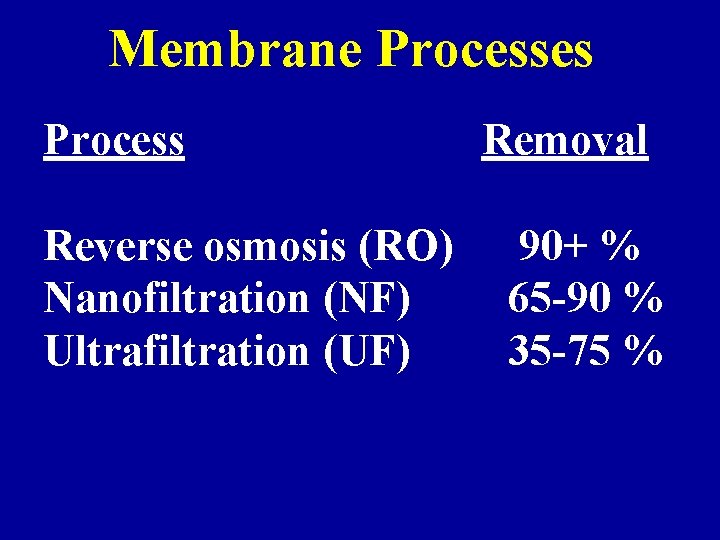
Membrane Processes Process Reverse osmosis (RO) Nanofiltration (NF) Ultrafiltration (UF) Removal 90+ % 65 -90 % 35 -75 %

Arsenic Removal Processes Large Systems Using Surface Waters • Coagulation/filtration • Direct filtration • Lime softening

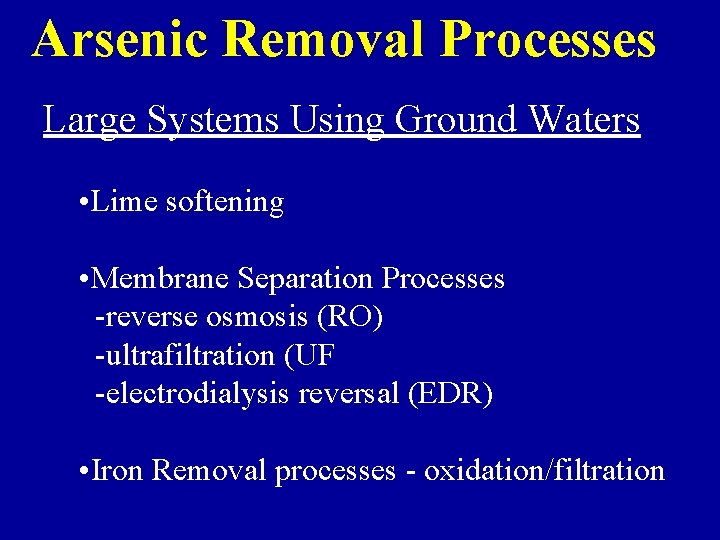
Arsenic Removal Processes Large Systems Using Ground Waters • Lime softening • Membrane Separation Processes -reverse osmosis (RO) -ultrafiltration (UF -electrodialysis reversal (EDR) • Iron Removal processes - oxidation/filtration

Arsenic Removal Processes Small Systems Using Surface Waters • Coagulation/filtration package plants • Iron Removal processes oxidation/filtration • Lime softening package plants

Arsenic Removal Processes Small Systems Using Ground Waters • Anion Exchange • Activated Alumina adsorption • Iron Removal processes - oxid/filt. • Membrane Separation Processes -reverse osmosis (RO) -ultrafiltration (UF) -electrodialysis reversal (EDR)

Arsenic Removal Processes Very Small Community Option • Point-of-use systems -RO, AA • Point-of-entry systems -RO, Ion Exchange

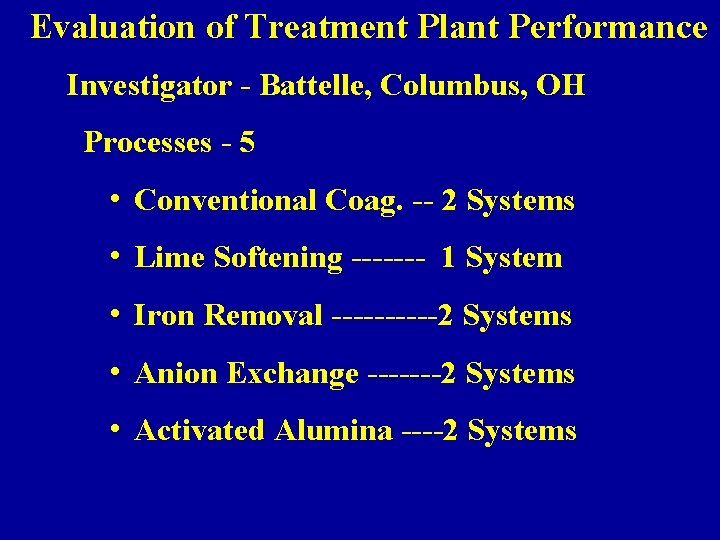
Evaluation of Treatment Plant Performance Investigator - Battelle, Columbus, OH Processes - 5 i Conventional Coag. -- 2 Systems i Lime Softening ------- 1 System i Iron Removal -----2 Systems i Anion Exchange -------2 Systems i Activated Alumina ----2 Systems

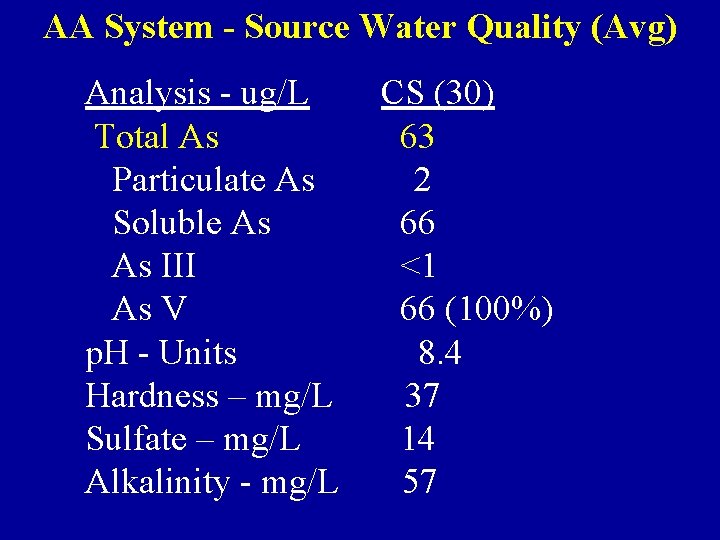
AA System - Source Water Quality (Avg) Analysis - ug/L Total As Particulate As Soluble As As III As V p. H - Units Hardness – mg/L Sulfate – mg/L Alkalinity - mg/L CS (30) 63 2 66 <1 66 (100%) 8. 4 37 14 57

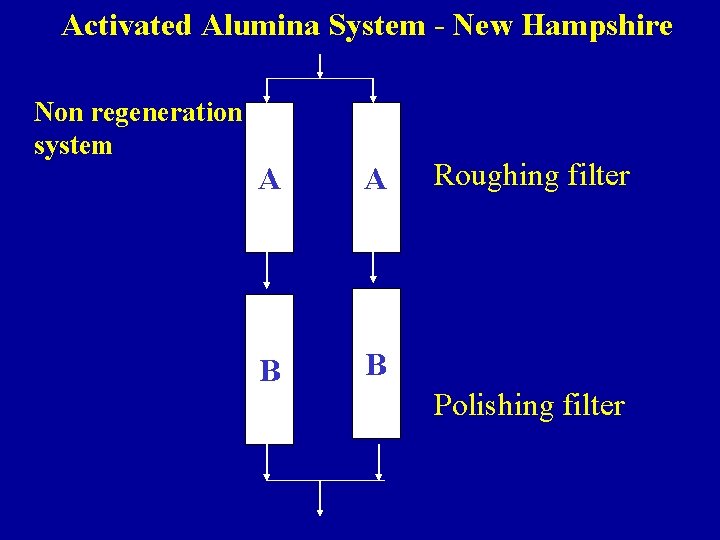
Activated Alumina System - New Hampshire Non regeneration system A A B B Roughing filter Polishing filter

Activated Alumina System, 20 gpm - NH

Activated Alumina System, NH

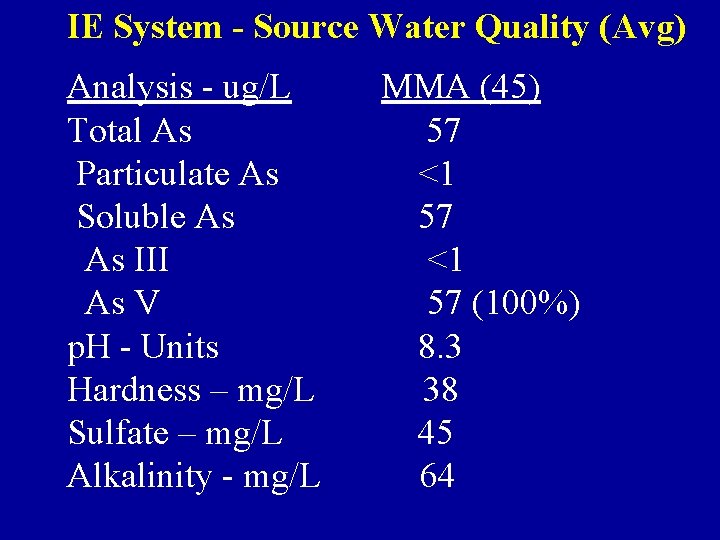
IE System - Source Water Quality (Avg) Analysis - ug/L Total As Particulate As Soluble As As III As V p. H - Units Hardness – mg/L Sulfate – mg/L Alkalinity - mg/L MMA (45) 57 <1 57 (100%) 8. 3 38 45 64

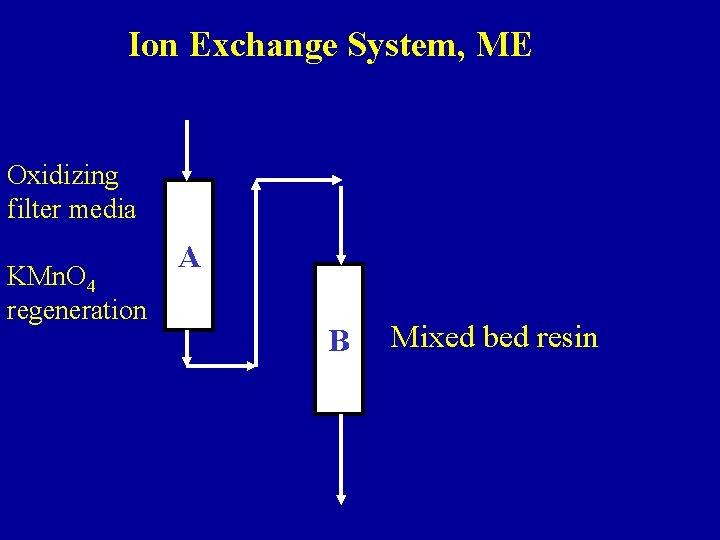
Ion Exchange System, ME Oxidizing filter media KMn. O 4 regeneration A B Mixed bed resin

Ion Exchange System with Oxidizing Filter, ME 2 gpm

Ion Exchange System, ME

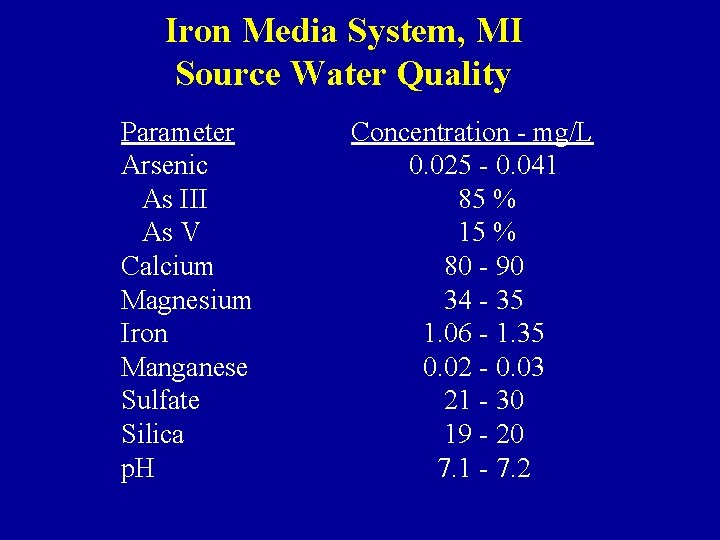
Iron Media System, MI Source Water Quality Parameter Arsenic As III As V Calcium Magnesium Iron Manganese Sulfate Silica p. H Concentration - mg/L 0. 025 - 0. 041 85 % 15 % 80 - 90 34 - 35 1. 06 - 1. 35 0. 02 - 0. 03 21 - 30 19 - 20 7. 1 - 7. 2

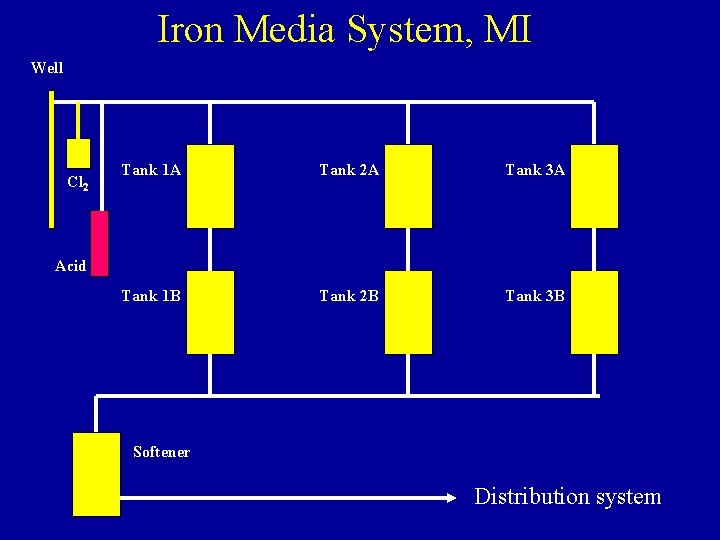
Iron Media System, MI Well Cl 2 Tank 1 A Tank 2 A Tank 3 A Tank 1 B Tank 2 B Tank 3 B Acid Softener Distribution system

Iron Media System, MI

Iron Media System, MI

SUMMARY • Soluble arsenic occurs in natural water in the As III and As V oxidation states. • As V is dominant in oxygenated waters • As III is dominant in anoxic water

SUMMARY • Treatment processes remove As V more effectively than As III • As III can be converted to As V with strong oxidants

SUMMARY • Most conventional treatment processes have capability to reduce arsenic to less than 10 ug/L, many to to 5 ug/L or less.

Tom Sorg USEPA Cincinnati, OH 45268 513 -569 -7370 sorg. thomas@epa. gov