Year 9 Science Matter Isotopes Learning Intentions Learning


- Slides: 13

Year 9 Science: Matter Isotopes

Learning Intentions Learning intention: To be able to explain the existence of isotopes as variations in the number of neutrons Success Criteria: Explain how isotopes of the same elements have different numbers of neutrons and thus different atomic masses Define relative atomic mass

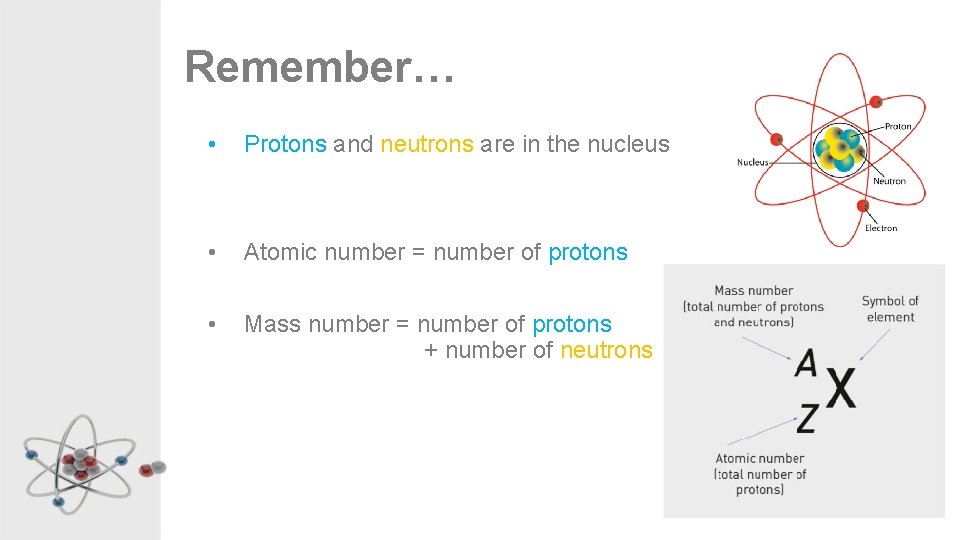
Remember… • Protons and neutrons are in the nucleus • Atomic number = number of protons • Mass number = number of protons + number of neutrons

Isotopes • Atoms that have the same number of protons, but different numbers of neutrons are called isotopes • In other words, isotopes have the same atomic number, but different mass numbers. • Since they have the same number of protons, isotopes are different forms of the same element.

Isotopes • In the picture, both are copper atoms, both have 29 protons, but have different numbers of neutrons and so weigh different amounts

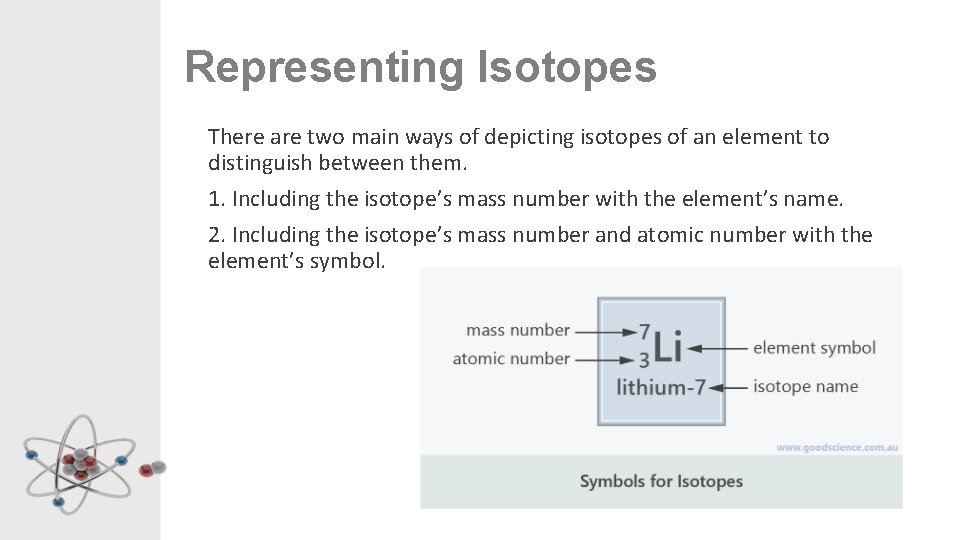
Representing Isotopes There are two main ways of depicting isotopes of an element to distinguish between them. 1. Including the isotope’s mass number with the element’s name. 2. Including the isotope’s mass number and atomic number with the element’s symbol.

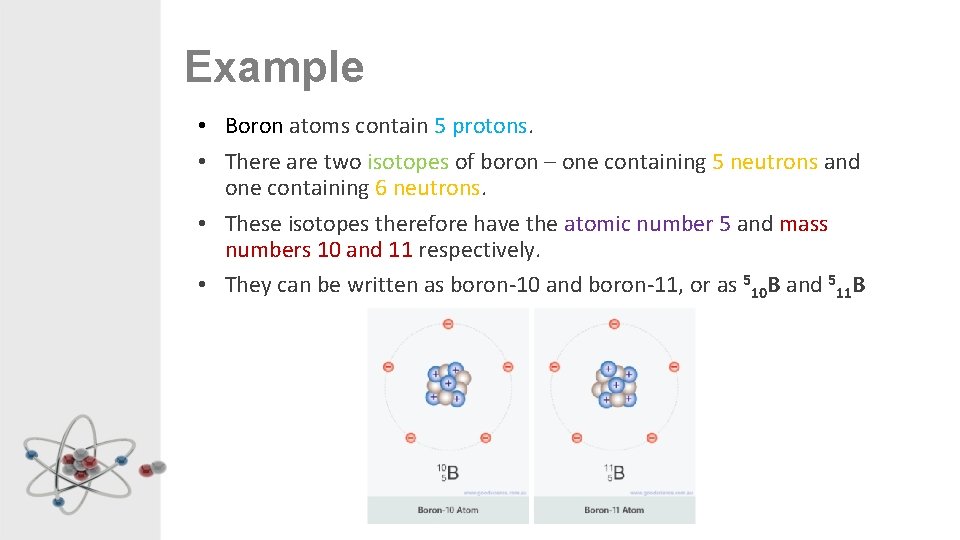
Example • Boron atoms contain 5 protons. • There are two isotopes of boron – one containing 5 neutrons and one containing 6 neutrons. • These isotopes therefore have the atomic number 5 and mass numbers 10 and 11 respectively. • They can be written as boron-10 and boron-11, or as 510 B and 511 B

Naturally Occurring Isotopes Almost all elements exist as two or more isotopes. The proportions of different isotopes are fixed for a particular element, but differ between elements. For example, • naturally occurring boron consists of • 20% boron-10 • 80% boron-11 • naturally occurring bromine consists of • 51% bromine-79 • 49% bromine-81

Relative Atomic Mass • On the Periodic Table, masses (weights) of elements aren’t (usually) whole numbers • This is because of isotopes • Elements don’t exist as single atoms, so the Periodic Table shows the mass as an average of the isotopes of that element • This is called the Relative Atomic Mass

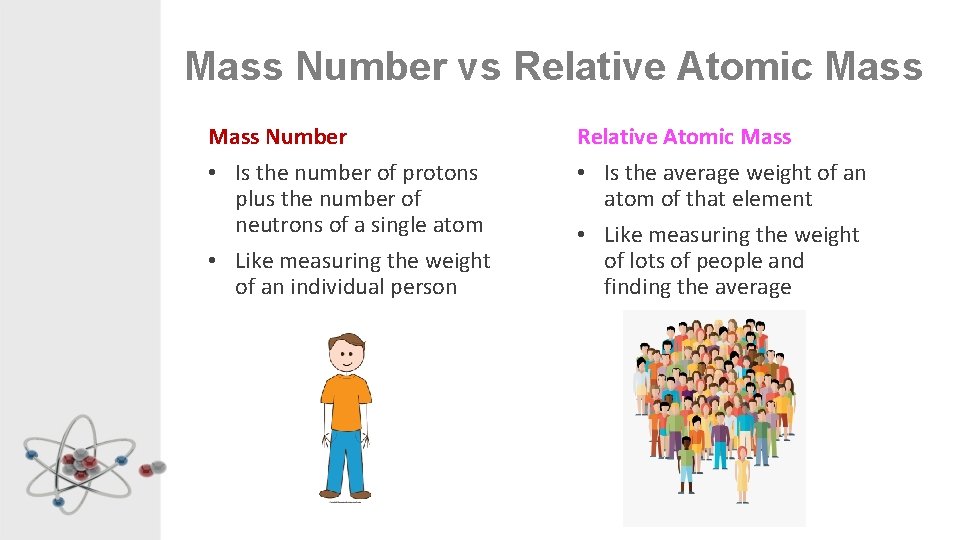
Mass Number vs Relative Atomic Mass Number • Is the number of protons plus the number of neutrons of a single atom • Like measuring the weight of an individual person Relative Atomic Mass • Is the average weight of an atom of that element • Like measuring the weight of lots of people and finding the average

Examples Lithium - 6 Atomic Number: Mass Number: Isotope Symbol: Lithium - 7 Atomic Number: Mass Number: Isotope Symbol: Lithium - 8 Atomic Number: Mass Number: Isotope Symbol: Number of protons: Number of neutrons: Number of electrons: Aluminium - 26 Atomic Number: Mass Number: Isotope Symbol: Aluminium - 27 Atomic Number: Mass Number: Isotope Symbol: Number of protons: Number of neutrons: Number of electrons:

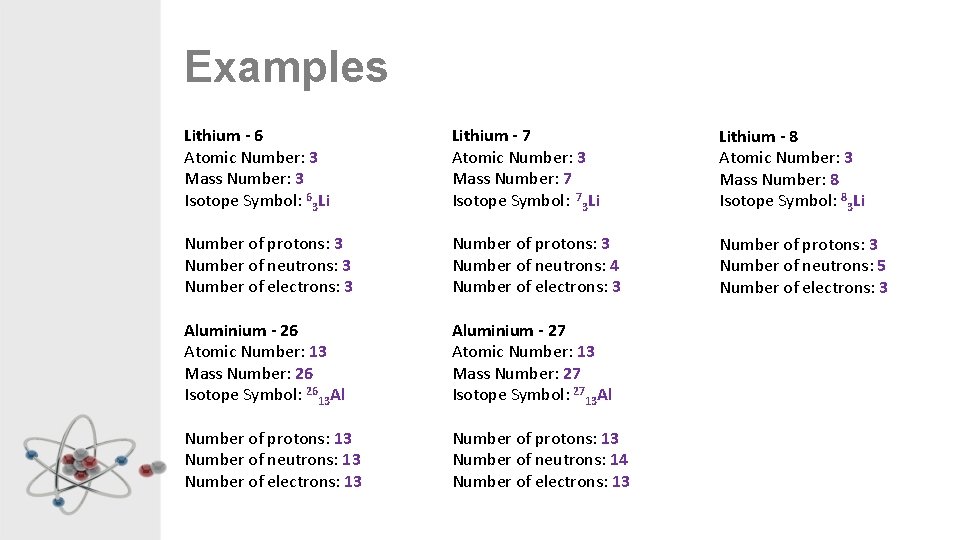
Examples Lithium - 6 Atomic Number: 3 Mass Number: 3 Isotope Symbol: 63 Li Lithium - 7 Atomic Number: 3 Mass Number: 7 Isotope Symbol: 73 Li Lithium - 8 Atomic Number: 3 Mass Number: 8 Isotope Symbol: 83 Li Number of protons: 3 Number of neutrons: 3 Number of electrons: 3 Number of protons: 3 Number of neutrons: 4 Number of electrons: 3 Number of protons: 3 Number of neutrons: 5 Number of electrons: 3 Aluminium - 26 Atomic Number: 13 Mass Number: 26 Isotope Symbol: 2613 Al Aluminium - 27 Atomic Number: 13 Mass Number: 27 Isotope Symbol: 2713 Al Number of protons: 13 Number of neutrons: 13 Number of electrons: 13 Number of protons: 13 Number of neutrons: 14 Number of electrons: 13

Activities • Complete Notes • Worksheet: Isotopes • Check Your Learning 5. 6 1, 3, 4