Environmental isotopes as tracers in geochemistry Environmental Isotopes









- Slides: 29

Environmental isotopes as tracers in geochemistry

Environmental Isotopes in Hydrogeology • Introduction to the environmental isotopes • Tracing the hydrologic cycle - 18 O, 2 H, 13 C • Tracing mineral systems - 18 O, 13 C, 34 S

Nucleosynthesis of the elements and isotopes + 1 H 2 H + b+ + v 2 H + 1 H 3 He + g 4 He + 4 He 8 Be + 4 He 12 C + g 12 C + 1 H 13 C + b+ + v + g 13 C + 1 H 14 N + g 14 N + 1 H 15 O + g 15 N + b+ + v 12 C + 4 He 16 O + 2 n 18 O. . . 1 H


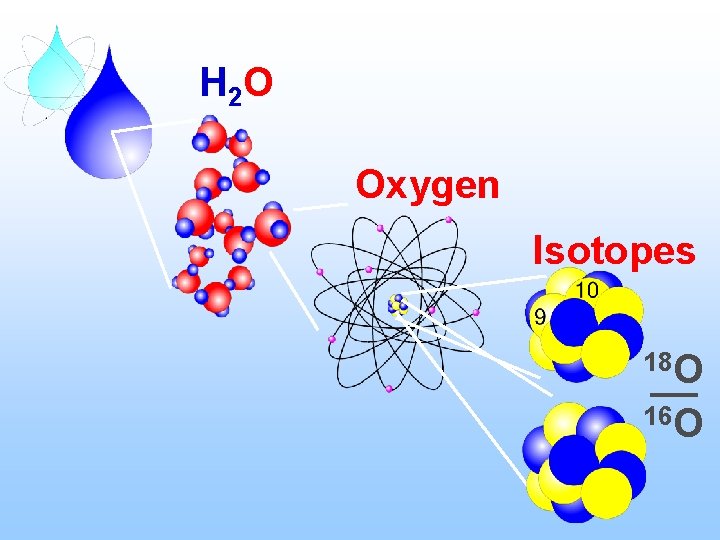
H 2 O Oxygen Isotopes 18 O 16 O

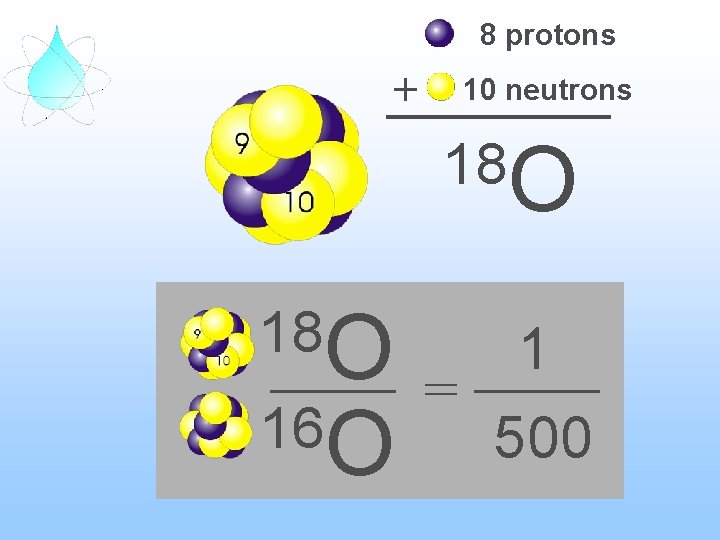
8 protons + 10 neutrons 18 O 16 O 1 = 500

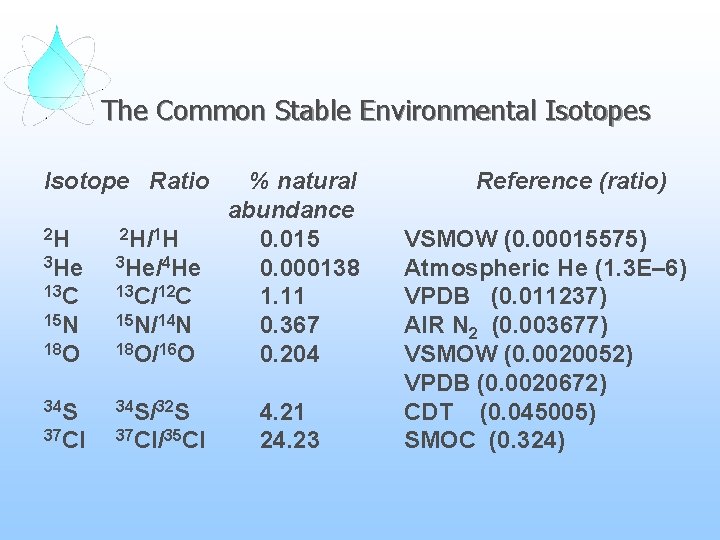
The Common Stable Environmental Isotopes Isotope Ratio 2 H 2 H/1 H 3 He/4 He 13 C/12 C 15 N/14 N 18 O/16 O 34 S/32 S 37 Cl/35 Cl % natural abundance 0. 015 0. 000138 1. 11 0. 367 0. 204 4. 21 24. 23 Reference (ratio) VSMOW (0. 00015575) Atmospheric He (1. 3 E– 6) VPDB (0. 011237) AIR N 2 (0. 003677) VSMOW (0. 0020052) VPDB (0. 0020672) CDT (0. 045005) SMOC (0. 324)

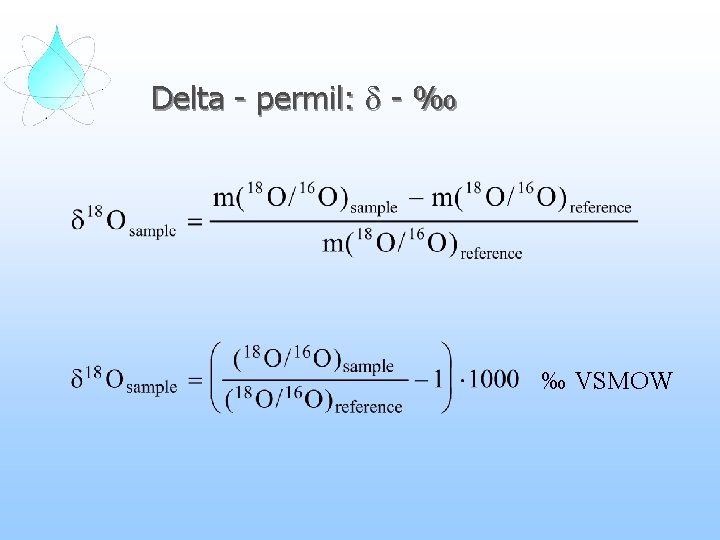
Delta - permil: d - ‰

Delta - permil: d - ‰ ‰ VSMOW

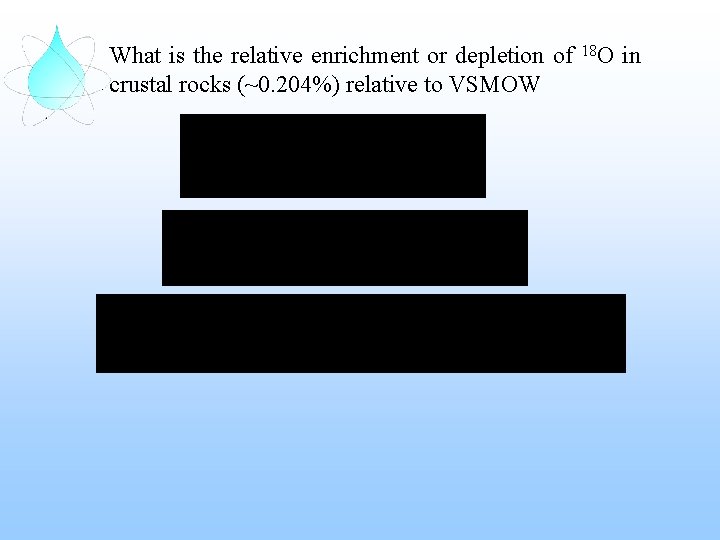
What is the relative enrichment or depletion of 18 O in crustal rocks (~0. 204%) relative to VSMOW

What is the relative enrichment or depletion of 18 O in crustal rocks (~0. 204%) relative to VSMOW

What is the relative enrichment or depletion of 18 O in crustal rocks (~0. 204%) relative to VSMOW

What is the relative enrichment or depletion of 18 O in crustal rocks (~0. 204%) relative to VSMOW = 17. 4‰ VSMOW crustal rocks are enriched in 18 O by 17. 4‰ or 1. 7% relative to the standard VSMOW

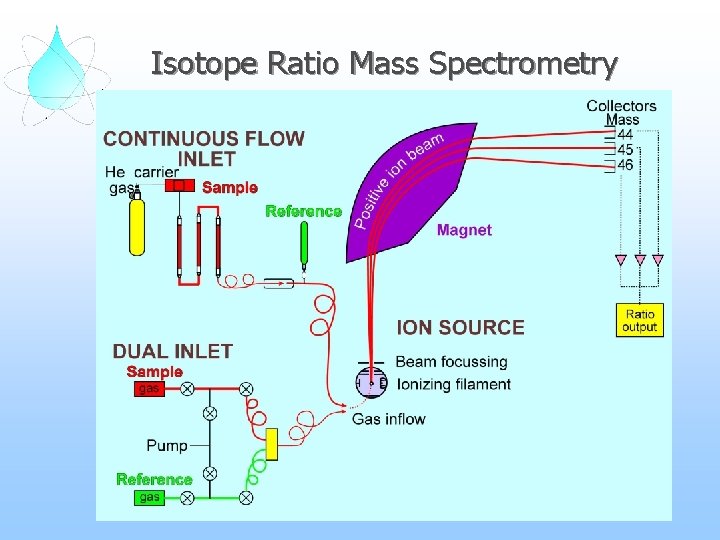
Isotope Ratio Mass Spectrometry

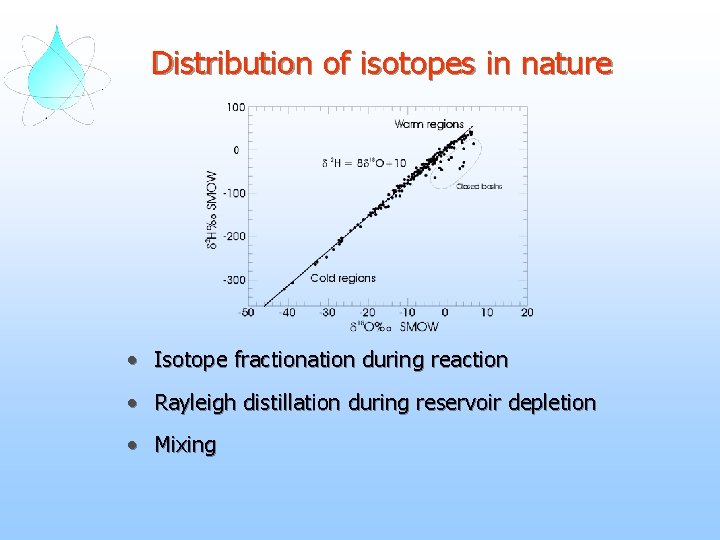
Distribution of isotopes in nature • Isotope fractionation during reaction • Rayleigh distillation during reservoir depletion • Mixing

Isotope fractionation, a

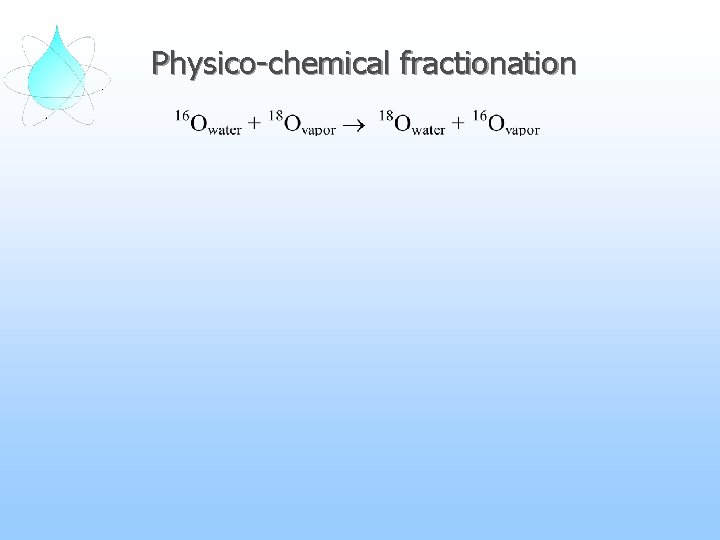
Physico-chemical fractionation

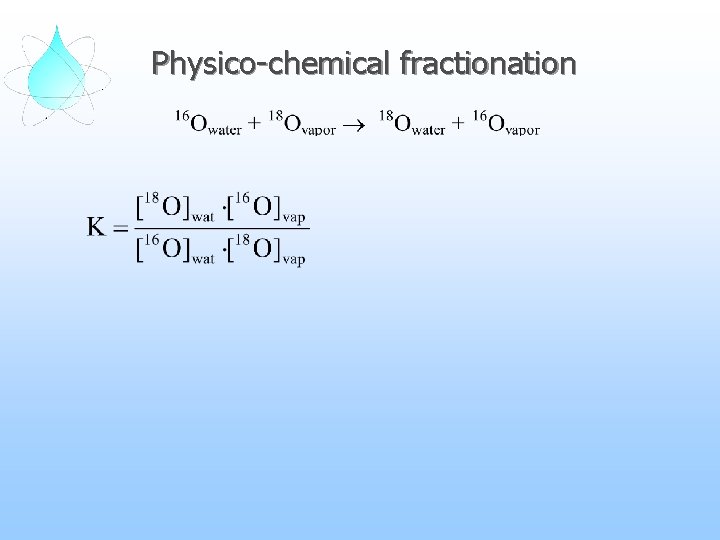
Physico-chemical fractionation

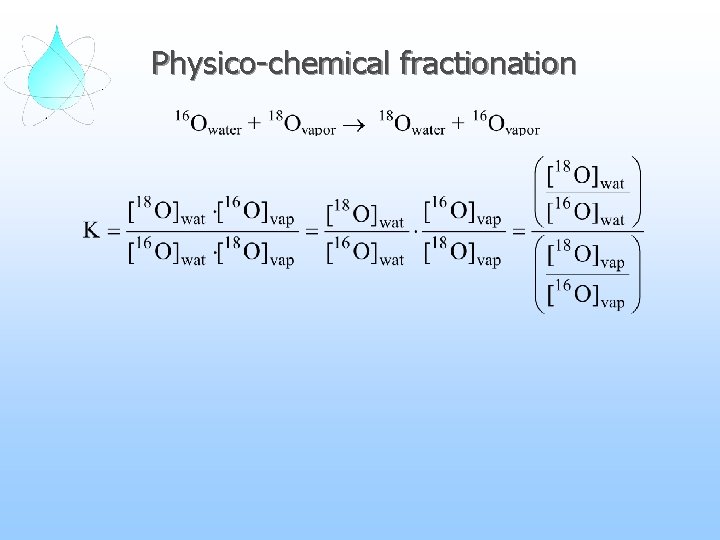
Physico-chemical fractionation

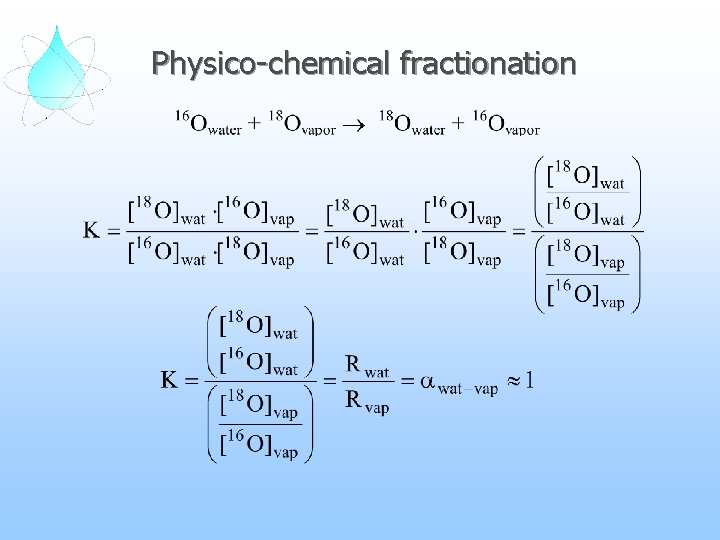
Physico-chemical fractionation

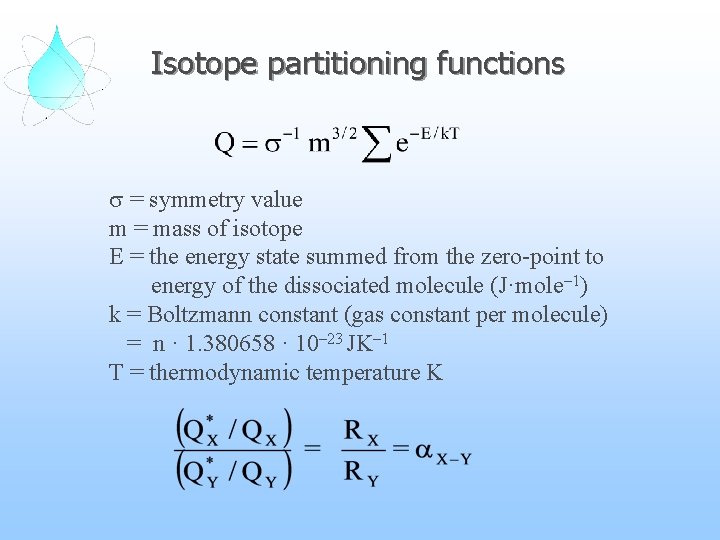
Isotope partitioning functions = symmetry value m = mass of isotope E = the energy state summed from the zero-point to energy of the dissociated molecule (J·mole– 1) k = Boltzmann constant (gas constant per molecule) = n · 1. 380658 · 10– 23 JK– 1 T = thermodynamic temperature K

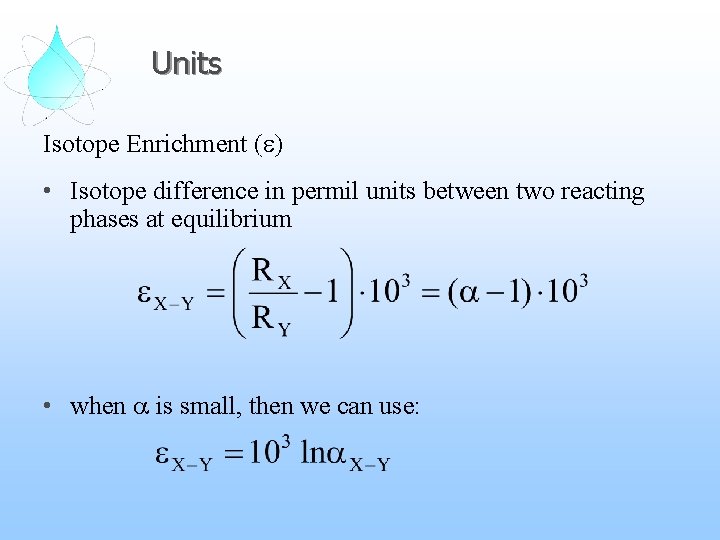
Units Isotope Enrichment (e) • Isotope difference in permil units between two reacting phases at equilibrium • when a is small, then we can use:

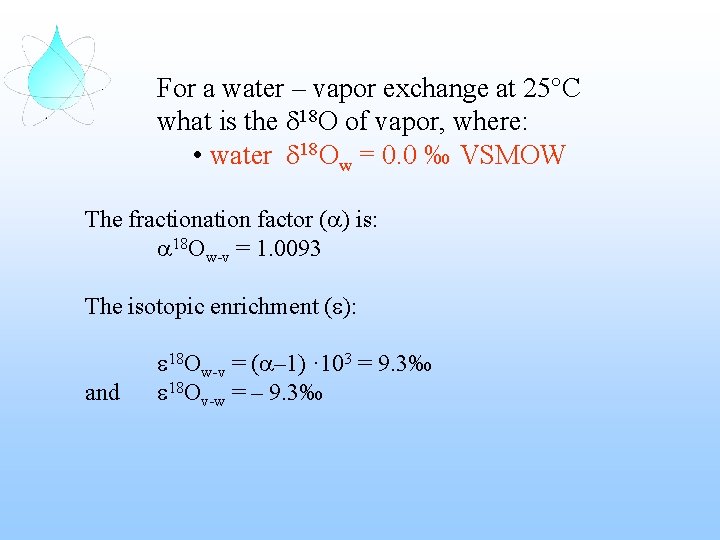
For a water – vapor exchange at 25°C what is the d 18 O of vapor, where: • water d 18 Ow = 0. 0 ‰ VSMOW

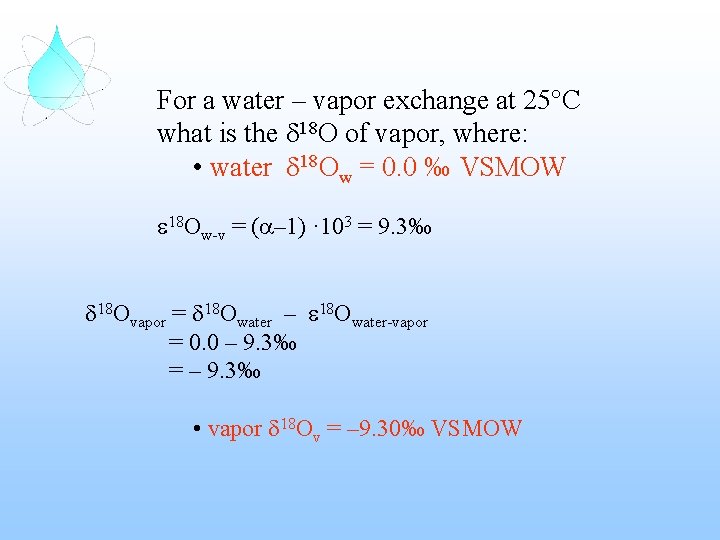
For a water – vapor exchange at 25°C what is the d 18 O of vapor, where: • water d 18 Ow = 0. 0 ‰ VSMOW The fractionation factor (a) is: a 18 Ow-v = 1. 0093 The isotopic enrichment (e): and e 18 Ow-v = (a– 1) · 103 = 9. 3‰ e 18 Ov-w = – 9. 3‰

For a water – vapor exchange at 25°C what is the d 18 O of vapor, where: • water d 18 Ow = 0. 0 ‰ VSMOW e 18 Ow-v = (a– 1) · 103 = 9. 3‰ d 18 Ovapor = d 18 Owater – e 18 Owater-vapor = 0. 0 – 9. 3‰ = – 9. 3‰ • vapor d 18 Ov = – 9. 30‰ VSMOW

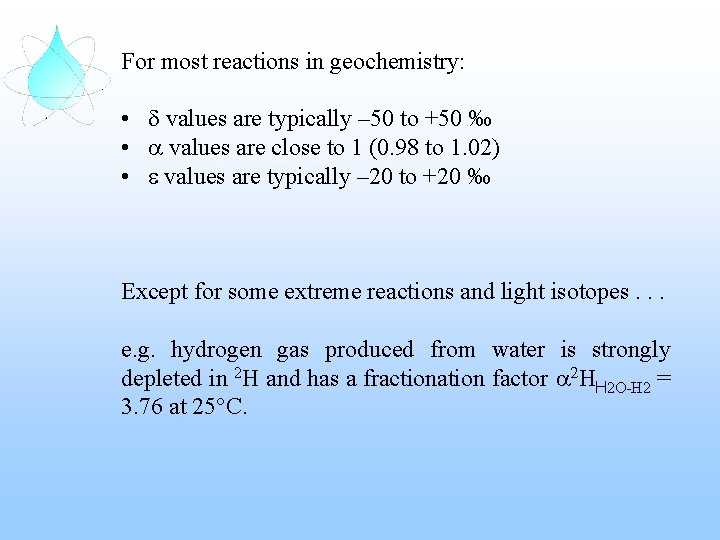
For most reactions in geochemistry: • d values are typically – 50 to +50 ‰ • a values are close to 1 (0. 98 to 1. 02) • e values are typically – 20 to +20 ‰ Except for some extreme reactions and light isotopes. . . e. g. hydrogen gas produced from water is strongly depleted in 2 H and has a fractionation factor a 2 HH 2 O-H 2 = 3. 76 at 25°C.

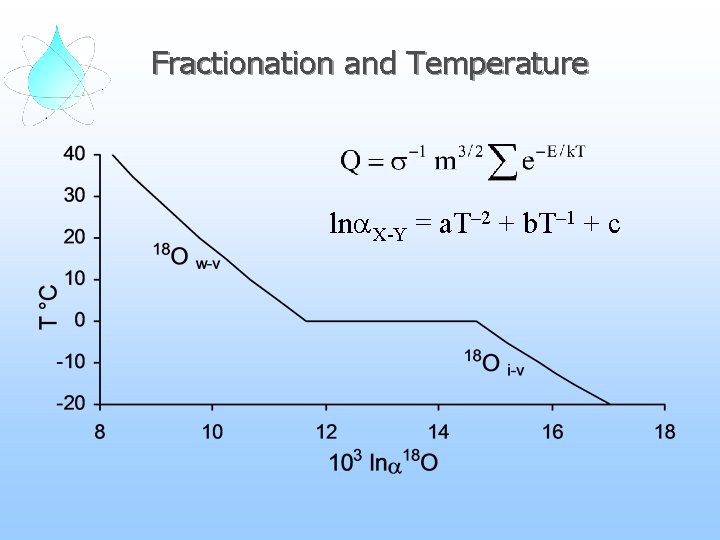
Fractionation and Temperature lna. X-Y = a. T– 2 + b. T– 1 + c

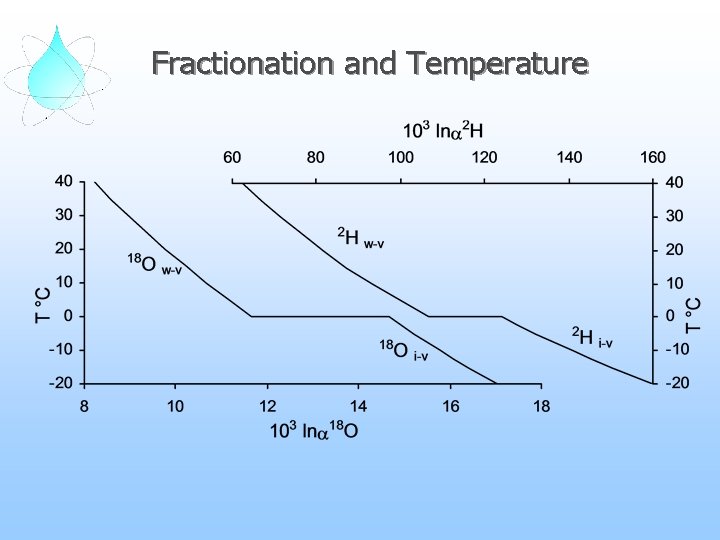
Fractionation and Temperature

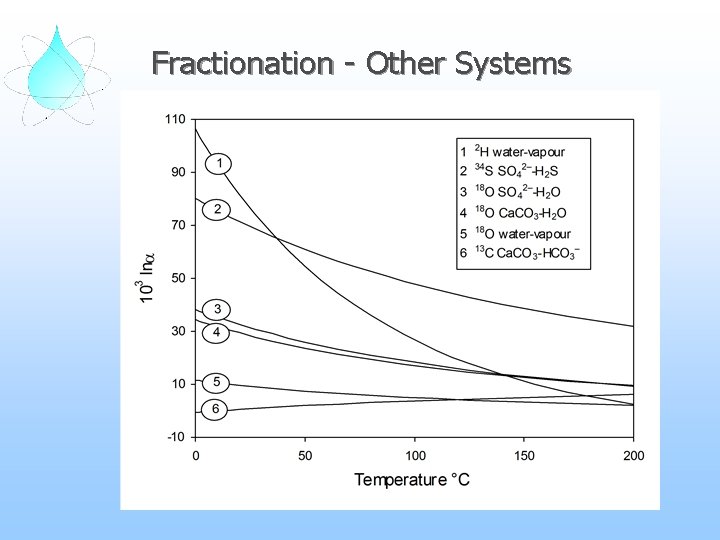
Fractionation - Other Systems
 Radioactive tracers in agriculture
Radioactive tracers in agriculture Radioactive tracers in agriculture
Radioactive tracers in agriculture What is geochemistry in geology
What is geochemistry in geology What is ionic radii
What is ionic radii Msc marine biogeochemistry
Msc marine biogeochemistry Isotopes pogil
Isotopes pogil Isotope abundance formula
Isotope abundance formula Isotopes examples
Isotopes examples Oxoacids of nitrogen
Oxoacids of nitrogen Rubidium orbital diagram
Rubidium orbital diagram Isotopes radioactifs
Isotopes radioactifs Subatomic heavyweights isotopes lesson 13
Subatomic heavyweights isotopes lesson 13 O
O A x z atom
A x z atom Isotopes properties
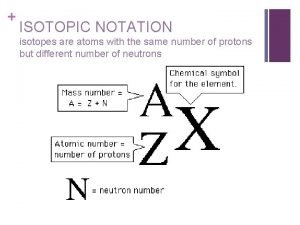
Isotopes properties Isotopic notation
Isotopic notation Four fundamental forces of nature
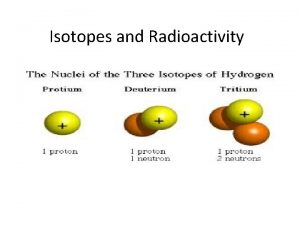
Four fundamental forces of nature Hydrogen isotopes
Hydrogen isotopes Stable vs unstable isotope
Stable vs unstable isotope Isotopes
Isotopes Northwest medical isotopes
Northwest medical isotopes Atomic isotopes
Atomic isotopes Examples of isotopes
Examples of isotopes Hydrogen isotopes
Hydrogen isotopes Hyphen notation of the three isotopes of hydrogen
Hyphen notation of the three isotopes of hydrogen What is mass number
What is mass number Fertile isotopes
Fertile isotopes Isotopes properties
Isotopes properties The isotope atoms differ in *
The isotope atoms differ in * Uses of radioactive isotopes
Uses of radioactive isotopes