Wood Chemistry PSE 406 Lecture 17 Chemical Isolation

















- Slides: 28

Wood Chemistry PSE 406 Lecture 17 Chemical Isolation and Analysis II Hemicelluloses and Lignin Analysis PSE 406 - Lecture 17 1

Class Agenda l l l How are hemicelluloses separated from cellulose and lignin? How are individual hemicelluloses separated? How is the composition of individual hemicelluloses determined? How are the linkages determined? Lignin analysis PSE 406 - Lecture 17 2

How are hemicelluloses separated from cellulose and lignin? l l Generate Holocelulose Remember…. in this procedure lignin is removed through the action of sodium chlorite PSE 406 - Lecture 17 3

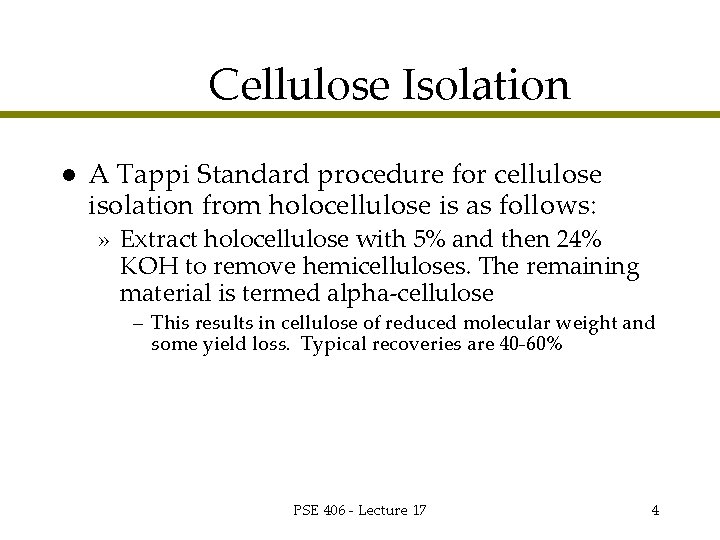
Cellulose Isolation l A Tappi Standard procedure for cellulose isolation from holocellulose is as follows: » Extract holocellulose with 5% and then 24% KOH to remove hemicelluloses. The remaining material is termed alpha-cellulose – This results in cellulose of reduced molecular weight and some yield loss. Typical recoveries are 40 -60% PSE 406 - Lecture 17 4

How are the hemicelluloses separated from cellulose? l l Cellulose is not soluble in almost any solvents. What are hemicelluloses soluble in? » Na. OH or KOH!!!!! PSE 406 - Lecture 17 5

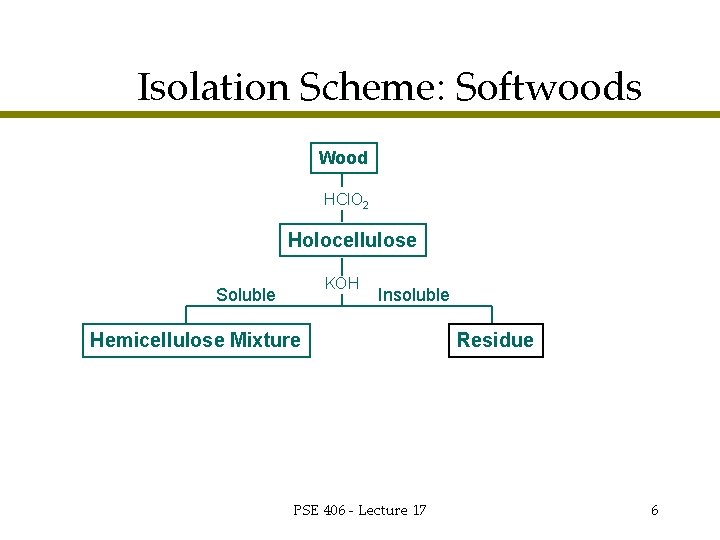
Isolation Scheme: Softwoods Wood HCl. O 2 Holocellulose KOH Soluble Insoluble Hemicellulose Mixture PSE 406 - Lecture 17 Residue 6

What is in the residue? l Cellulose » It is not soluble in much of anything l Galactoglucomannan (not the water soluble) » It turns out that this hemicellulose is not all that alkali soluble at this level of KOH » It takes the addition of Na. OH and borate to solublize this material PSE 406 - Lecture 17 7

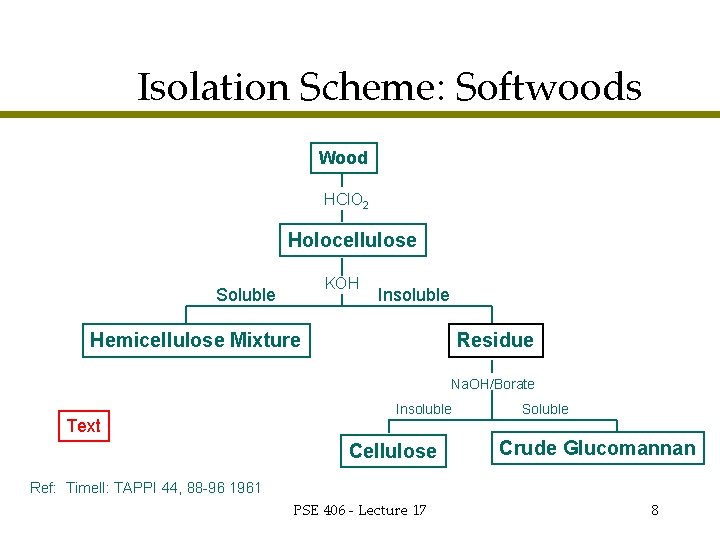
Isolation Scheme: Softwoods Wood HCl. O 2 Holocellulose KOH Soluble Insoluble Hemicellulose Mixture Residue Na. OH/Borate Text Insoluble Cellulose Soluble Crude Glucomannan Ref: Timell: TAPPI 44, 88 -96 1961 PSE 406 - Lecture 17 8

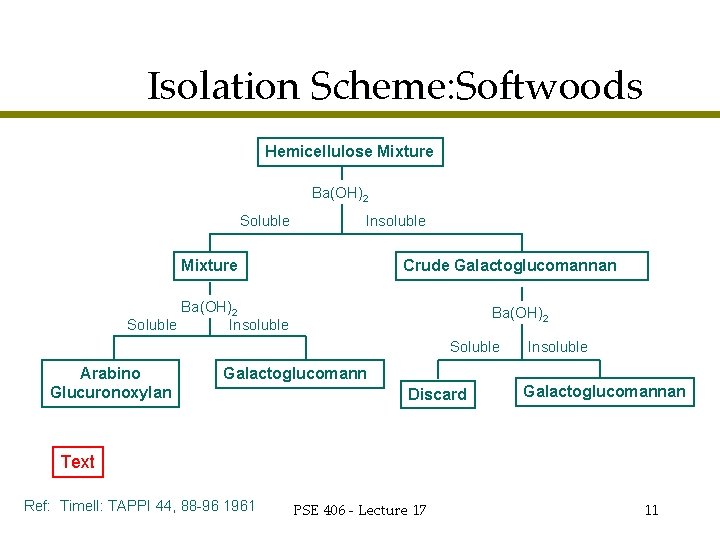
What makes up the rest of the hemicellulose mixture? l l Xylans Galactoglucomannans (water soluble) Maybe some pectins, a little glucans, and who know what else We are mainly concerned with the top two…. » How do we separate the xylans from the galactoglucomannans? PSE 406 - Lecture 17 9

Barium l l Because of the orientation of the C 2 and C 3 hydroxyl groups in mannose, it will form an insoluble complex with barium ions. Therefore the addition of Ba(OH)2 will cause glucomannans to precipitate out of solution PSE 406 - Lecture 17 10

Isolation Scheme: Softwoods Hemicellulose Mixture Ba(OH)2 Soluble Insoluble Crude Galactoglucomannan Mixture Ba(OH)2 Soluble Insoluble Ba(OH)2 Soluble Arabino Glucuronoxylan Insoluble Galactoglucomann Discard Galactoglucomannan Text Ref: Timell: TAPPI 44, 88 -96 1961 PSE 406 - Lecture 17 11

How is the composition of individual hemicelluloses determined? l How can hemicelluloses be broken down into individual sugars. » Acid hydrolysis of glycosidic linkages. » Enzymatic hydrolysis PSE 406 - Lecture 17 12

Hemicellulose Analysis l The individual sugars are quantified using gas or liquid chromatography. » Often the individual components require derivitization before analysis. » Other analytical techniques are used to positively identify components PSE 406 - Lecture 17 13

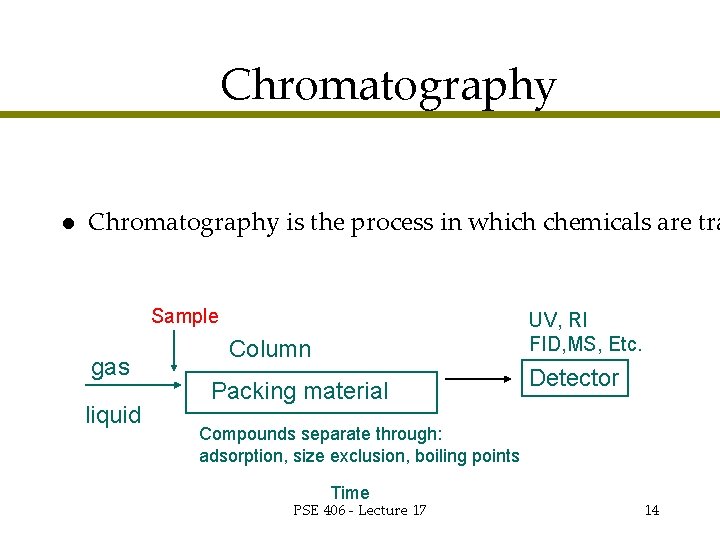
Chromatography l Chromatography is the process in which chemicals are tra Sample gas liquid UV, RI FID, MS, Etc. Column Packing material Detector Compounds separate through: adsorption, size exclusion, boiling points Time PSE 406 - Lecture 17 14

Derivitization l l l Gas Chromatography - Chemicals to be analyzed must be volatile: Sugars and uronic acids are not volatile. Blocking hydroxyl groups will make chemicals volatile. Derivitization procedures: » Methylation » Acetylation » Silylation PSE 406 - Lecture 17 15

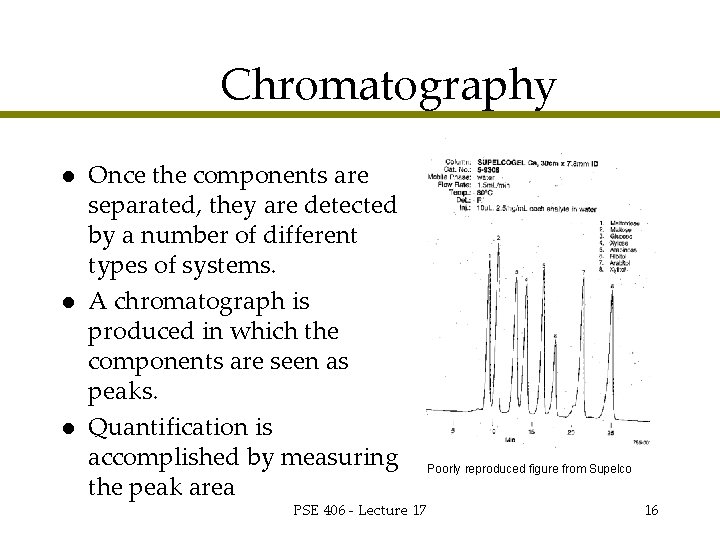
Chromatography l l l Once the components are separated, they are detected by a number of different types of systems. A chromatograph is produced in which the components are seen as peaks. Quantification is accomplished by measuring the peak area PSE 406 - Lecture 17 Poorly reproduced figure from Supelco 16

Determination of Linkages How is it possible to determine how the individual sugars are linked? l Methylation of the free hydoxyl groups l Acid Hydrolysis l Chromatographic determination of products l PSE 406 - Lecture 17 17

Pyranose? Furanose? l Mild hydrolysis of hemicellulose results in the presence of monomers, dimers, trimers, etc. of the hemicelluloses. » These materials can be separated by chromatography and compared to known dimers. PSE 406 - Lecture 17 18

Lignin-Important Questions l How much lignin is in a sample? » Wood » Plant Material » Pulp PSE 406 - Lecture 17 19

Quantification of Lignin l Wood and non-woody materials » Acid Insoluble lignin (along with acid soluble lig) l Pulp » Kappa number l Other non woody materials (or I don’t have a large sample to work with) » Acetyl bromide PSE 406 - Lecture 17 20

Acid Insoluble Lignin (Klason) l Goal is separate carbohydrates from lignin » Lignin condenses (reacts with lignin) to become very water insoluble (it becomes very large) » Acid cleaves glycosidic linkages in carbohydrates forming individual sugars. » Sugars dissolve in water (acid) and lignin does not PSE 406 - Lecture 17 21

Klason Procedure l Wood meal (or pulp) is treated with 72% H 2 SO 4 for 2 hours. The material is then diluted to 3% H 2 SO 4 and then boiled for 4 hours. » These two steps dissolves the carbohydrates leaving chunks of lignin floating in the acid l The lignin is filtered, washed and weighed. PSE 406 - Lecture 17 22

Acid Soluble Lignin l A certain percentage of the lignin is soluble in the Klason lignin procedure » This amount is very small with softwoods but higher >5% in hardwoods and grasses. l The filtrate from the Klason procedure is collected and the UV absorbance is checked. » Lignin absorbs UV light, sugars do not » The amount of lignin that is soluble is determined through comparing the UV absorbance to a standard. PSE 406 - Lecture 17 23

Lignin Content of Pulp l l Pulps contain only small amounts of lignin so a different (and quicker) method is used: the kappa number. This procedure is based upon the fact that lignin reacts very quickly with KMn. O 4 while carbohydrates (mostly) react very slowly. PSE 406 - Lecture 17 24

Kappa Number Procedure l Pulp is dissolved in water and reacted with a KMn. O 4 solution for 10 minutes under very controlled conditions. » The goal is to consume 50% of the KMn. O 4 in this time. l l Excess KMn. O 4 is consumed with potassium iodide forming I 2 (iodine). The iodine is titrated with sodium thiosulfate to a starch endpoint. PSE 406 - Lecture 17 25

Kappa Number Information l l l This method is typically used with pulp containing low amounts of lignin (chemical unbleached pulp). It was found about 15 years ago that hexenuronic acids formed during kraft pulping from uronic acids consume KMn. O 4 thus giving false kappa numbers (if based only on lignin). A typical kappa number for an unbleached kraft pulp is around 20. PSE 406 - Lecture 17 26

Kappa to Klason l l Correction factors have been developed to convert kappa numbers to Klason lignin. These factors are different for different processes and species. Kraft pulps: % Klason = kappa number * 0. 15 Sulfite pulps: % Klason = kappa number * 0. 167 (or 0. 187 depending on who did the work). Kappa number 20 = ~3% lignin PSE 406 - Lecture 17 27

Acetyl Bromide Procedure l l This procedure was developed to measure lignin content in small samples. Samples are dissolved by reaction with acetyl bromide (with a little perchloric acid) in acetic acid. The solution is analyzed by UV (remember lignin absorbs, carbohydrates do not). The amount of lignin in the sample is determined by comparison against standards. » Every material seems to have a different standard number. PSE 406 - Lecture 17 28
 Pse wood meaning
Pse wood meaning Pse wood meaning
Pse wood meaning Softwood anatomy
Softwood anatomy Aerial stem modification examples
Aerial stem modification examples Comparative wood anatomy
Comparative wood anatomy Wood sawed wood old tongue twister
Wood sawed wood old tongue twister Wood wood teenager
Wood wood teenager 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad 406 woodruff key dimensions
406 woodruff key dimensions Despertai vos levantai vos
Despertai vos levantai vos Luo shu
Luo shu Ece 406
Ece 406 Ece 406
Ece 406 406 afsb
406 afsb Pharm 406
Pharm 406 401 stitch type
401 stitch type 406 registration
406 registration Cs 406
Cs 406 Nec article 406
Nec article 406 Hisense rb 406 n 4 ac 2
Hisense rb 406 n 4 ac 2 Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Is chopping firewood a physical or chemical change
Is chopping firewood a physical or chemical change Hammering wood together physical or chemical
Hammering wood together physical or chemical Physical and chemical changes
Physical and chemical changes Separating sand from gravel chemical or physical change
Separating sand from gravel chemical or physical change Is a bike rusting a chemical change
Is a bike rusting a chemical change Crumple physical or chemical change
Crumple physical or chemical change Is hammering wood together a physical change
Is hammering wood together a physical change