Wood Chemistry PSE 406Chem E 470 Lecture 18






- Slides: 17

Wood Chemistry PSE 406/Chem E 470 Lecture 18 Chemical Isolation and Analysis II Hemicelluloses PSE 406 - Lecture 17 1

Wood Chemistry l l Class Agenda How are hemicelluloses separated from cellulose and lignin? How are individual hemicelluloses separated? How is the composition of individual hemicelluloses determined? How are the linkages determined? PSE 406 - Lecture 17 2

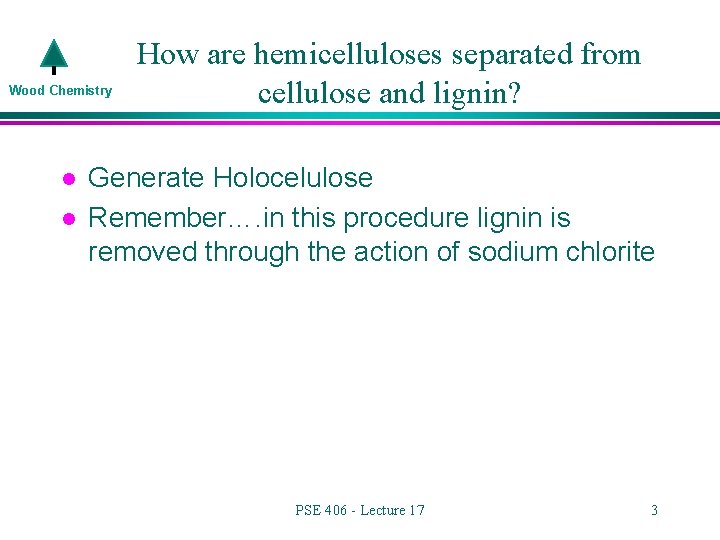
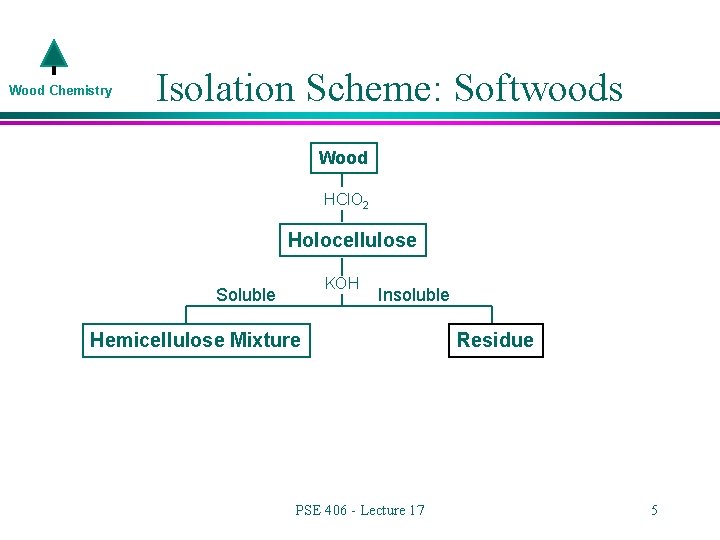
Wood Chemistry l l How are hemicelluloses separated from cellulose and lignin? Generate Holocelulose Remember…. in this procedure lignin is removed through the action of sodium chlorite PSE 406 - Lecture 17 3

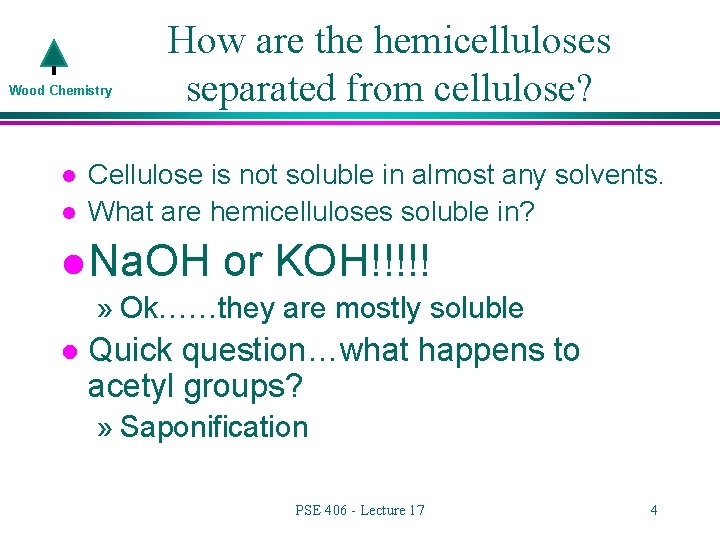
Wood Chemistry l l How are the hemicelluloses separated from cellulose? Cellulose is not soluble in almost any solvents. What are hemicelluloses soluble in? l Na. OH or KOH!!!!! » Ok……they are mostly soluble l Quick question…what happens to acetyl groups? » Saponification PSE 406 - Lecture 17 4

Wood Chemistry Isolation Scheme: Softwoods Wood HCl. O 2 Holocellulose KOH Soluble Insoluble Hemicellulose Mixture PSE 406 - Lecture 17 Residue 5

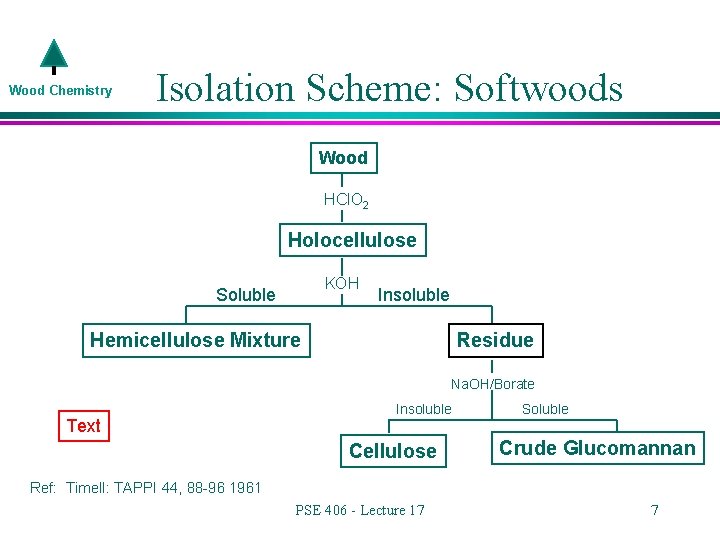
Wood Chemistry l What is in the residue? Cellulose » It is not soluble in much of anything l Galactoglucomannan (not the water soluble one) » It turns out that this hemicellulose is not all that alkali soluble at this level of KOH » It takes the addition of Na. OH and borate to solublize this material PSE 406 - Lecture 17 6

Wood Chemistry Isolation Scheme: Softwoods Wood HCl. O 2 Holocellulose KOH Soluble Insoluble Hemicellulose Mixture Residue Na. OH/Borate Text Insoluble Cellulose Soluble Crude Glucomannan Ref: Timell: TAPPI 44, 88 -96 1961 PSE 406 - Lecture 17 7

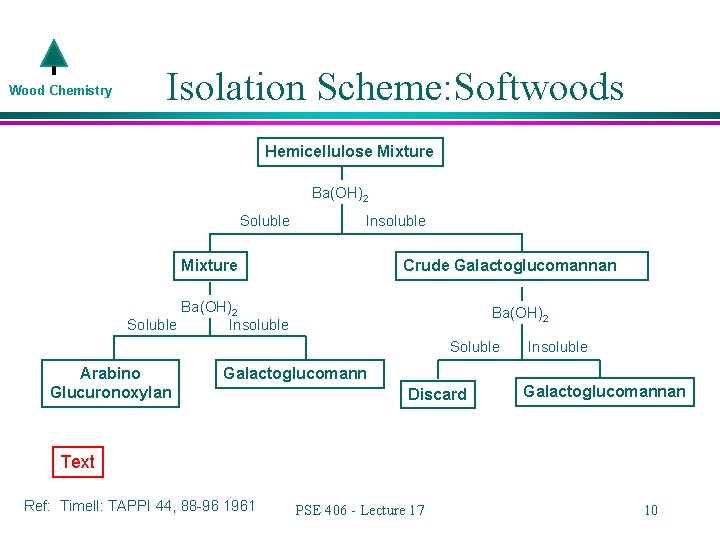
Wood Chemistry l l What makes up the rest of the hemicellulose mixture? Xylans Galactoglucomannans (water soluble) Maybe some pectins, a little glucans, and who know what else We are mainly concerned with the top two…. » How do we separate the xylans from the galactoglucomannans? PSE 406 - Lecture 17 8

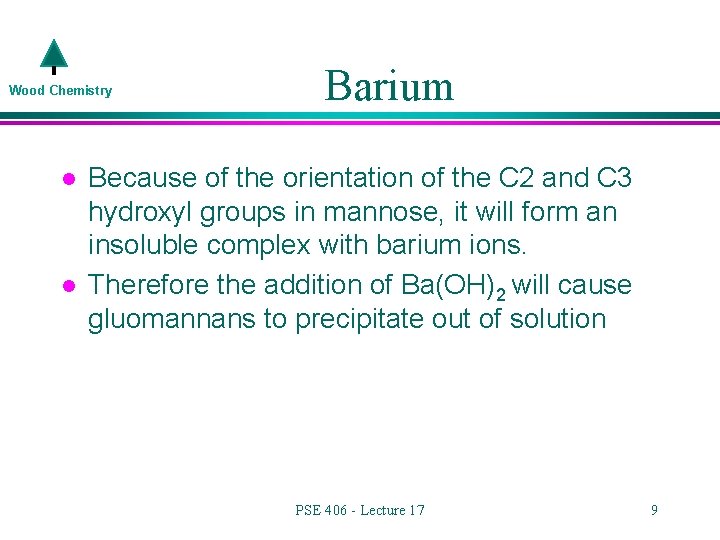
Wood Chemistry l l Barium Because of the orientation of the C 2 and C 3 hydroxyl groups in mannose, it will form an insoluble complex with barium ions. Therefore the addition of Ba(OH)2 will cause gluomannans to precipitate out of solution PSE 406 - Lecture 17 9

Wood Chemistry Isolation Scheme: Softwoods Hemicellulose Mixture Ba(OH)2 Soluble Insoluble Crude Galactoglucomannan Mixture Ba(OH)2 Soluble Insoluble Ba(OH)2 Soluble Arabino Glucuronoxylan Insoluble Galactoglucomann Discard Galactoglucomannan Text Ref: Timell: TAPPI 44, 88 -96 1961 PSE 406 - Lecture 17 10

Wood Chemistry l How is the composition of individual hemicelluloses determined? How can hemicelluloses be broken down into individual sugars. l Acid hydrolysis of glycosidic linkages !!!!!!! PSE 406 - Lecture 17 11

Wood Chemistry l Hemicellulose Analysis The individual sugars are quantified using gas or liquid chromatography. » Often the individual components require derivitization before analysis. » Other analytical techniques are used to positively identify components PSE 406 - Lecture 17 12

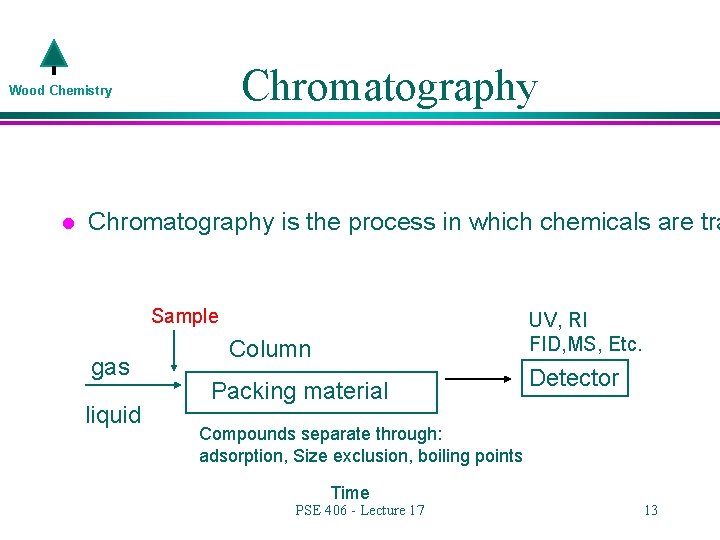
Chromatography Wood Chemistry l Chromatography is the process in which chemicals are tra Sample gas liquid UV, RI FID, MS, Etc. Column Packing material Detector Compounds separate through: adsorption, Size exclusion, boiling points Time PSE 406 - Lecture 17 13

Wood Chemistry l l l Derivitization Gas Chromatography - Chemicals to be analyzed must be volatile: Sugars and uronic acids are not volatile. Blocking hydroxyl groups will make chemicals volatile. Derivitization procedures: » Methylation » Acetylation » Silylation PSE 406 - Lecture 17 14

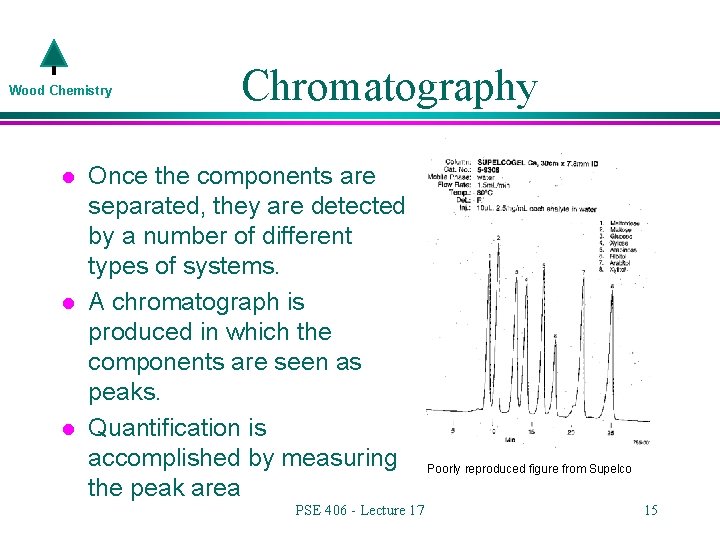
Wood Chemistry l l l Chromatography Once the components are separated, they are detected by a number of different types of systems. A chromatograph is produced in which the components are seen as peaks. Quantification is accomplished by measuring the peak area PSE 406 - Lecture 17 Poorly reproduced figure from Supelco 15

Wood Chemistry l l l Determination of Linkages How is it possible to determine how the individual sugars are linked? Methylation of the free hydoxyl groups Acid Hydrolysis Chromatographic determination of products Another method to determine linkages is the Smith degradation which involves periodate oxidation, borohydride reduction and very mild acid hydrolysis. We are not going to cover this reaction. PSE 406 - Lecture 17 16

Wood Chemistry l Pyranose? Furanose? Mild hydrolysis of hemicellulose results in the presence of monomers, dimers, trimers, etc of the hemicelluloses. » These materials can be separated by chromatography and compared to known dimers. PSE 406 - Lecture 17 17
 Pse wood meaning
Pse wood meaning Pse wood meaning
Pse wood meaning Esau wood poem
Esau wood poem Longitudinal tracheids
Longitudinal tracheids Wood wood teenager
Wood wood teenager Roots vegetative propagation
Roots vegetative propagation Angiosperm wood vs gymnosperm wood
Angiosperm wood vs gymnosperm wood 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Eecs 470
Eecs 470 470/12
470/12 Cs 470
Cs 470 470 399
470 399 470 クラスルール
470 クラスルール Log 470
Log 470 Cs 470
Cs 470 Mean median mode range math is fun
Mean median mode range math is fun Eecs 470
Eecs 470 Cs 470
Cs 470