Wood Chemistry PSE 406 Lecture 2 Monosaccharides PSE


- Slides: 19

Wood Chemistry PSE 406 Lecture 2: Monosaccharides PSE 406 - Lecture 2 1

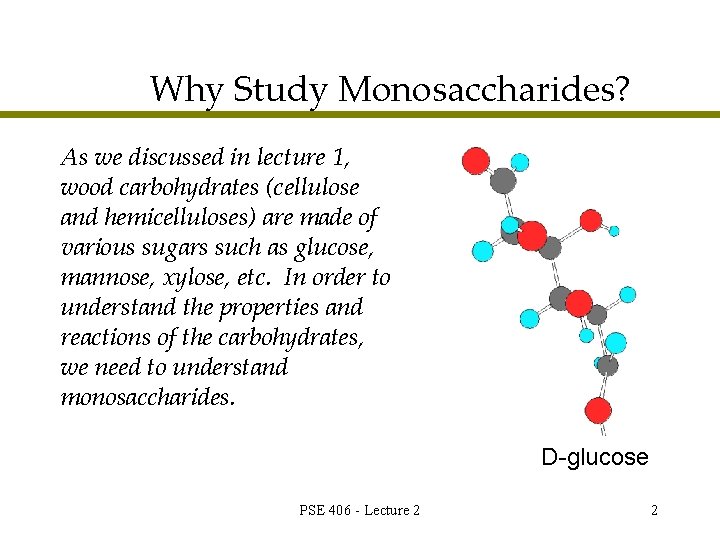
Why Study Monosaccharides? As we discussed in lecture 1, wood carbohydrates (cellulose and hemicelluloses) are made of various sugars such as glucose, mannose, xylose, etc. In order to understand the properties and reactions of the carbohydrates, we need to understand monosaccharides. D-glucose PSE 406 - Lecture 2 2

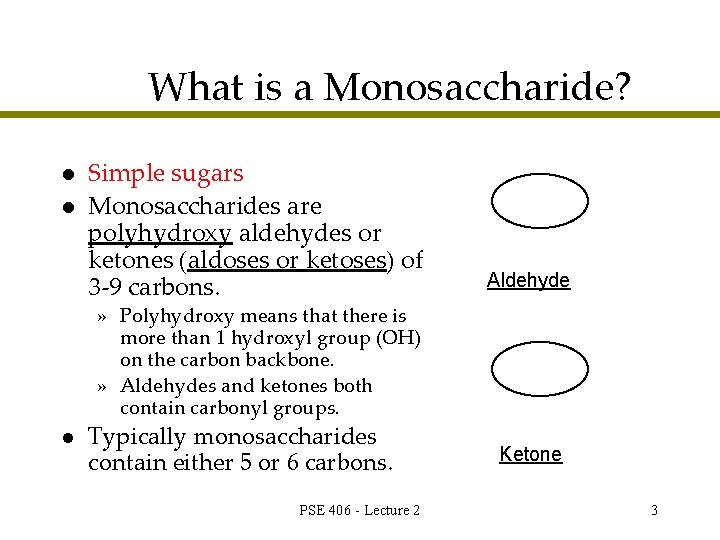
What is a Monosaccharide? l l Simple sugars Monosaccharides are polyhydroxy aldehydes or ketones (aldoses or ketoses) of 3 -9 carbons. Aldehyde » Polyhydroxy means that there is more than 1 hydroxyl group (OH) on the carbon backbone. » Aldehydes and ketones both contain carbonyl groups. l Typically monosaccharides contain either 5 or 6 carbons. PSE 406 - Lecture 2 Ketone 3

Glyceraldehyde l This 3 carbon sugar is the simplest monosaccharide. » It is an aldehyde. » As is the case with all monosaccharides, it contains an asymmetric or chiral carbon. This means there are 4 different groups attached to this carbon » Because of this, there are 2 different stereoisomers of this structure known as enantiomers. PSE 406 - Lecture 2 4

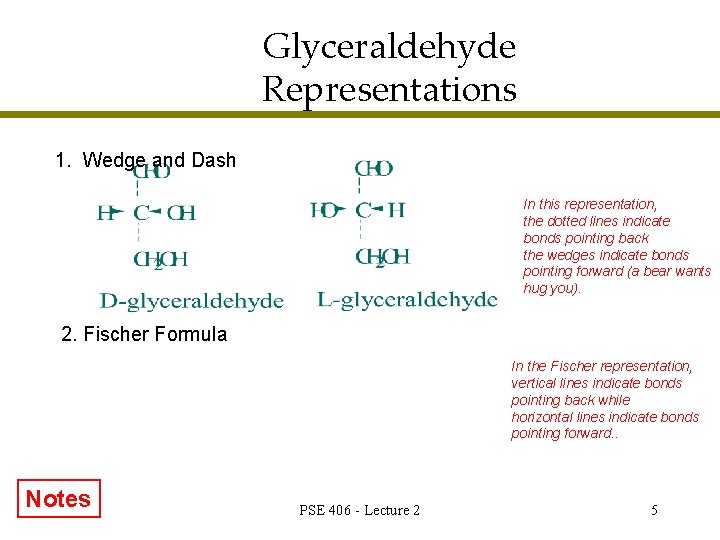
Glyceraldehyde Representations 1. Wedge and Dash In this representation, the dotted lines indicate bonds pointing back the wedges indicate bonds pointing forward (a bear wants hug you). 2. Fischer Formula In the Fischer representation, vertical lines indicate bonds pointing back while horizontal lines indicate bonds pointing forward. . Notes PSE 406 - Lecture 2 5

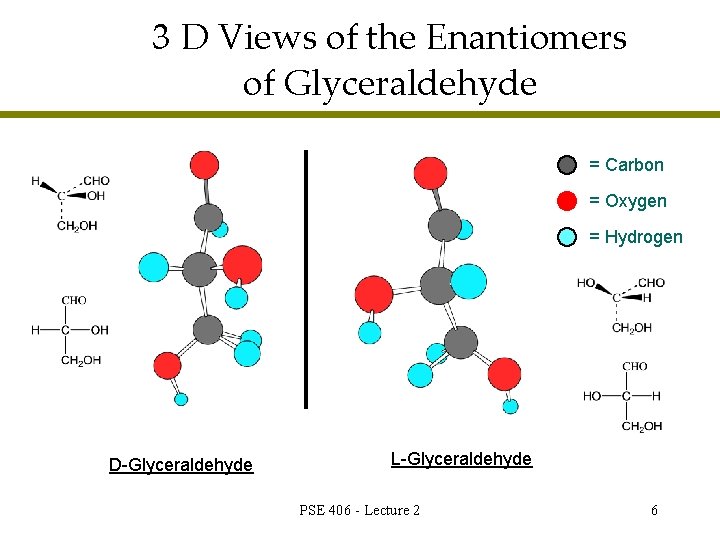
3 D Views of the Enantiomers of Glyceraldehyde = Carbon = Oxygen = Hydrogen D-Glyceraldehyde L-Glyceraldehyde PSE 406 - Lecture 2 6

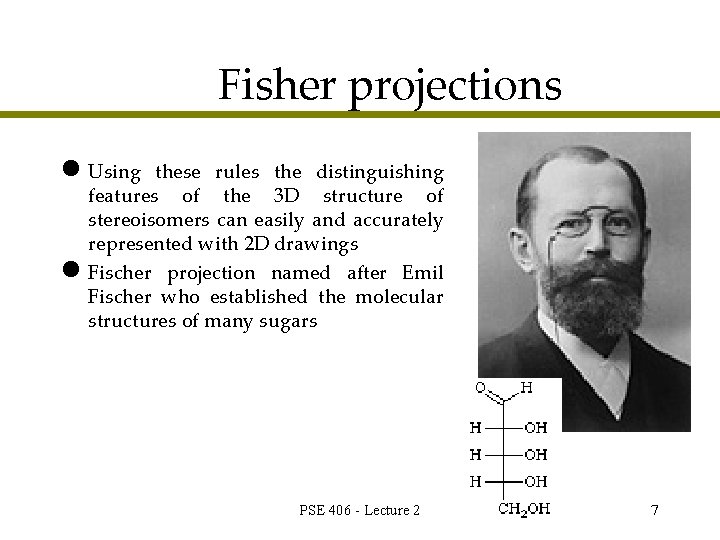
Fisher projections l Using these rules the distinguishing features of the 3 D structure of stereoisomers can easily and accurately represented with 2 D drawings l Fischer projection named after Emil Fischer who established the molecular structures of many sugars PSE 406 - Lecture 2 7

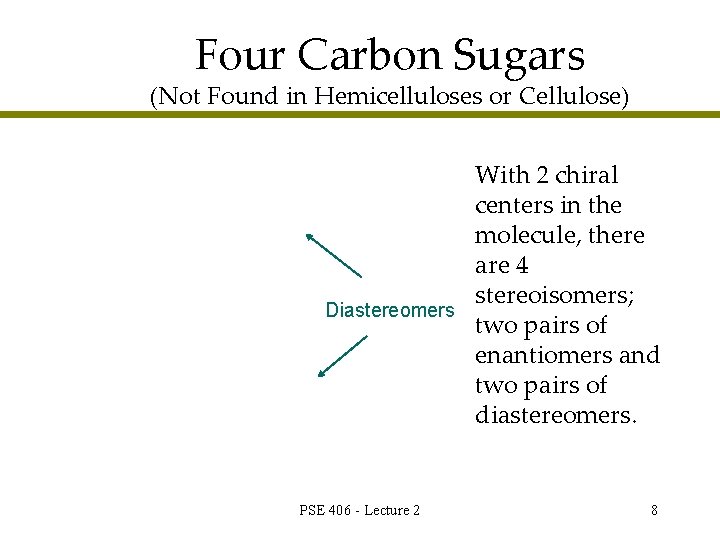
Four Carbon Sugars (Not Found in Hemicelluloses or Cellulose) Diastereomers PSE 406 - Lecture 2 With 2 chiral centers in the molecule, there are 4 stereoisomers; two pairs of enantiomers and two pairs of diastereomers. 8

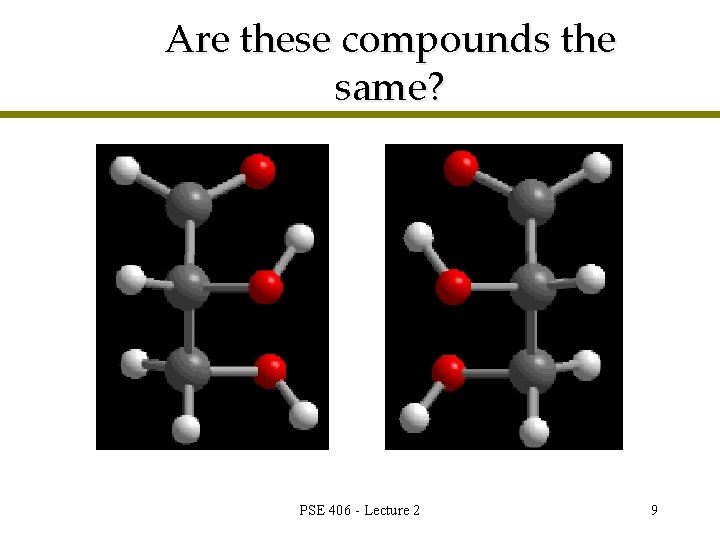
Are these compounds the same? PSE 406 - Lecture 2 9

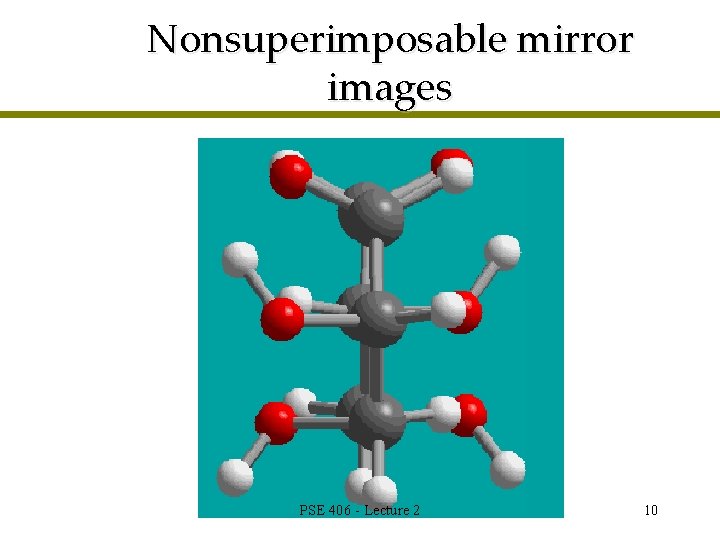
Nonsuperimposable mirror images PSE 406 - Lecture 2 10

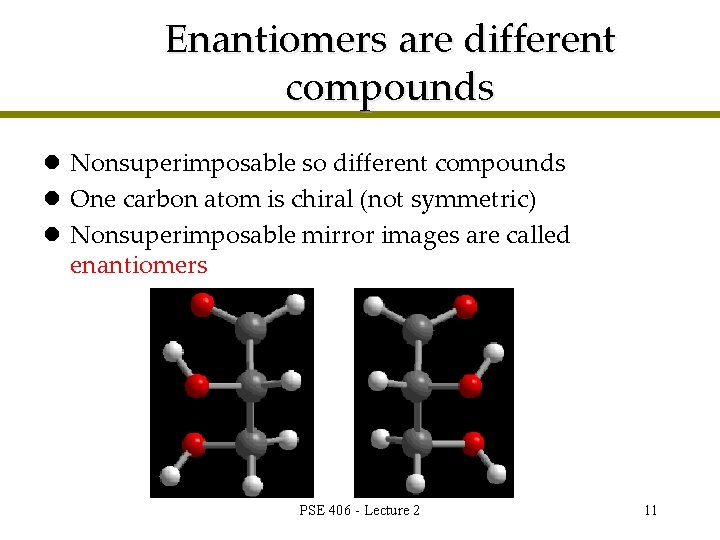
Enantiomers are different compounds l Nonsuperimposable so different compounds l One carbon atom is chiral (not symmetric) l Nonsuperimposable mirror images are called enantiomers PSE 406 - Lecture 2 11

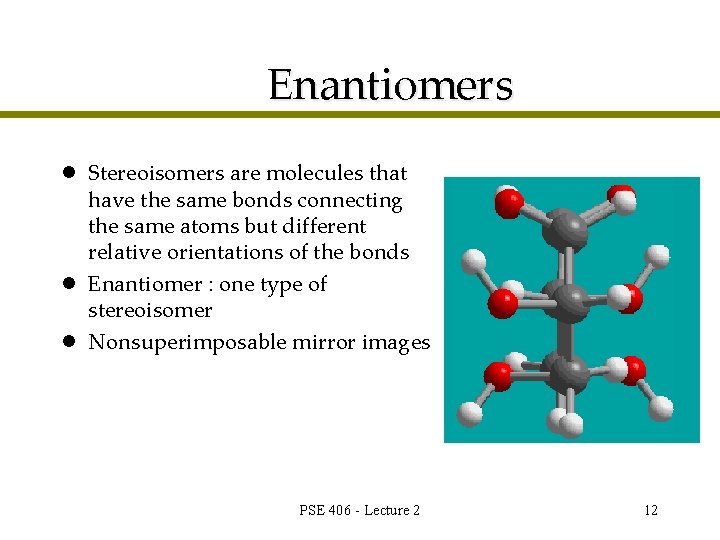
Enantiomers l Stereoisomers are molecules that have the same bonds connecting the same atoms but different relative orientations of the bonds l Enantiomer : one type of stereoisomer l Nonsuperimposable mirror images PSE 406 - Lecture 2 12

Stereochemistry l l l Enantiomers are identical in physical properties except that they rotate polarized light in opposite directions. From a biochemical standpoint, they are different (Nonsuperimposable mirror images). Diastereoisomers possess different physical properties (melting point, solubility) and often undergo chemical reaction in the different fashion. For n asymetric carbons there are: » 2 n maximum possible number of stereoisomers » 2 n-1 maximum possible number of enantiometric pairs » That means there are 2 n-1 possible diastereoisomer pairs PSE 406 - Lecture 2 13

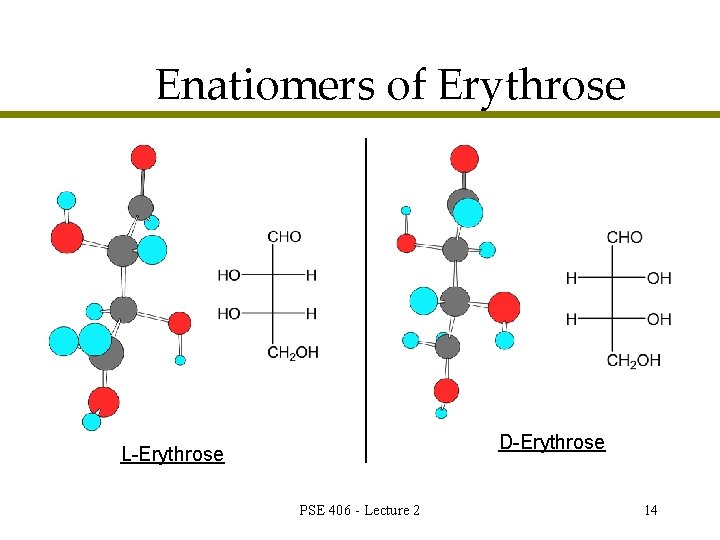
Enatiomers of Erythrose D-Erythrose L-Erythrose PSE 406 - Lecture 2 14

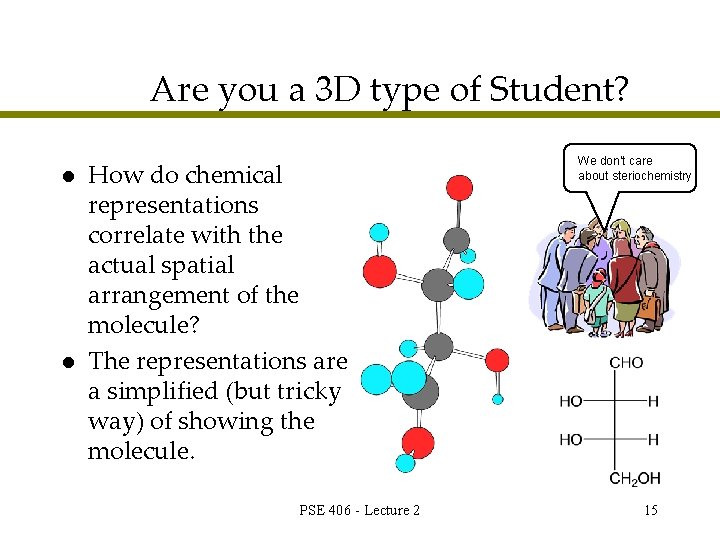
Are you a 3 D type of Student? l l How do chemical representations correlate with the actual spatial arrangement of the molecule? The representations are a simplified (but tricky way) of showing the molecule. PSE 406 - Lecture 2 We don’t care about steriochemistry 15

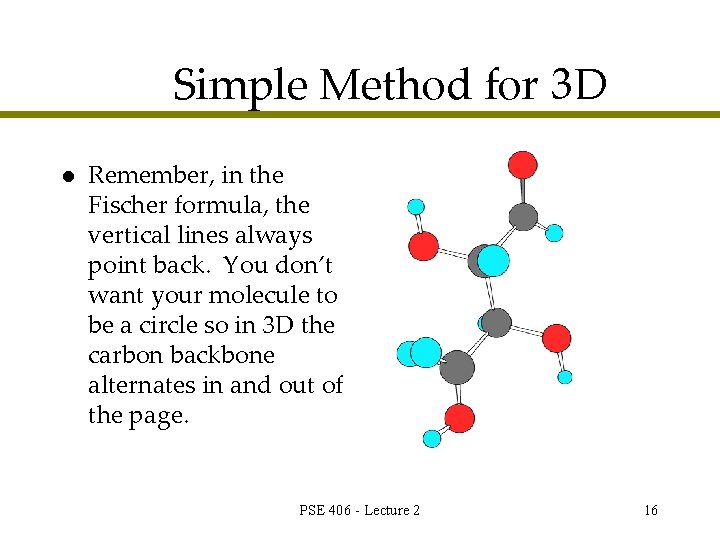
Simple Method for 3 D l Remember, in the Fischer formula, the vertical lines always point back. You don’t want your molecule to be a circle so in 3 D the carbon backbone alternates in and out of the page. PSE 406 - Lecture 2 16

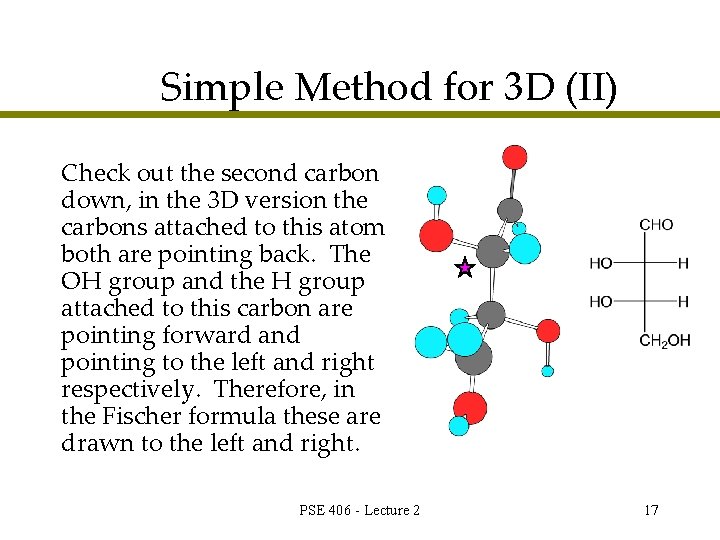
Simple Method for 3 D (II) Check out the second carbon down, in the 3 D version the carbons attached to this atom both are pointing back. The OH group and the H group attached to this carbon are pointing forward and pointing to the left and right respectively. Therefore, in the Fischer formula these are drawn to the left and right. PSE 406 - Lecture 2 17

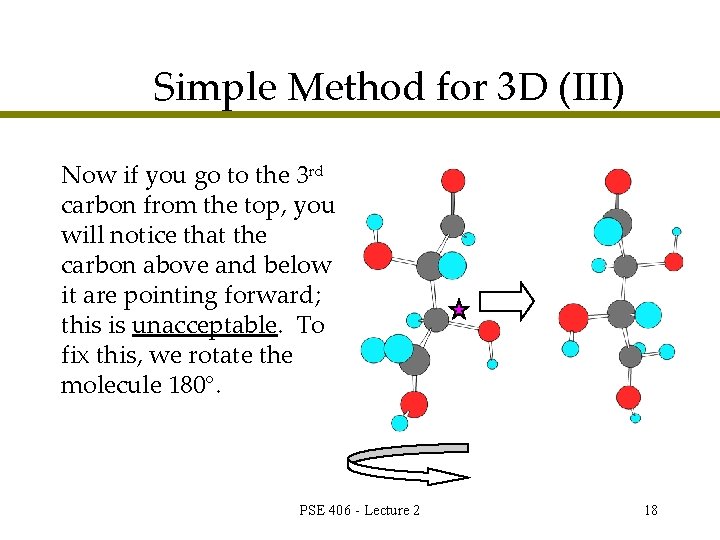
Simple Method for 3 D (III) Now if you go to the 3 rd carbon from the top, you will notice that the carbon above and below it are pointing forward; this is unacceptable. To fix this, we rotate the molecule 180°. PSE 406 - Lecture 2 18

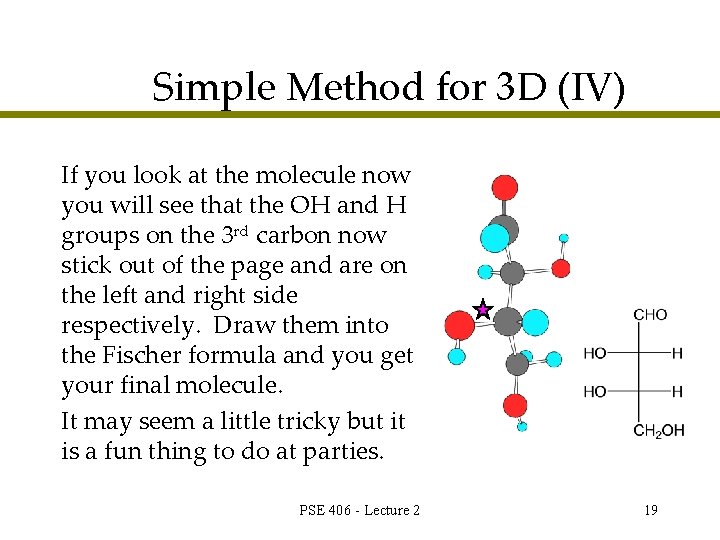
Simple Method for 3 D (IV) If you look at the molecule now you will see that the OH and H groups on the 3 rd carbon now stick out of the page and are on the left and right side respectively. Draw them into the Fischer formula and you get your final molecule. It may seem a little tricky but it is a fun thing to do at parties. PSE 406 - Lecture 2 19