Wood Chemistry PSE 406 Lecture 4 More Sugars






- Slides: 19

Wood Chemistry PSE 406 Lecture 4 More Sugars: Disaccharides and Rings PSE 406 - Lecture 4 1

Hemiacetal Formation A hemiacetal is a compound having the C(OH)OR group that results from the reaction of an aldehyde with one mole of an alcohol. Sugar molecules contain an aldehyde group and lots of alcohol groups. PSE 406 - Lecture 4 2

Hemiacetal Formation in Sugars (1) Notes PSE 406 - Lecture 4 3

Hemiacetal Formation in Sugars (2) β-D-glucopyranose α-D-glucopyranose PSE 406 - Lecture 4 4

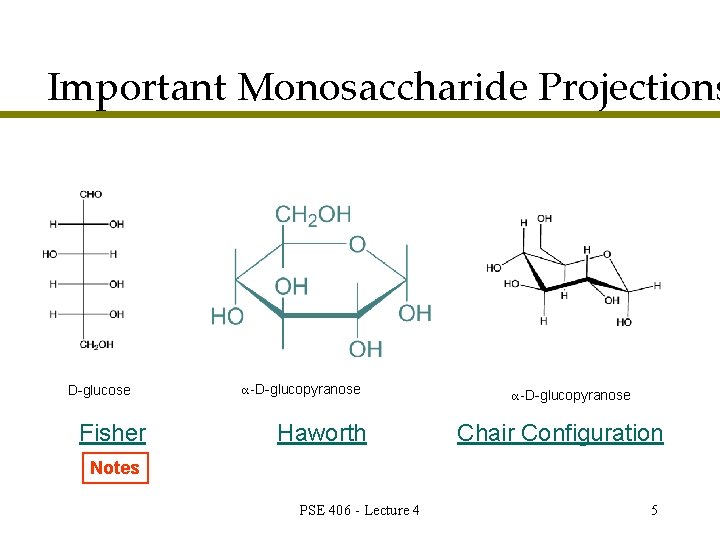
Important Monosaccharide Projections D-glucose Fisher α-D-glucopyranose Haworth α-D-glucopyranose Chair Configuration Notes PSE 406 - Lecture 4 5

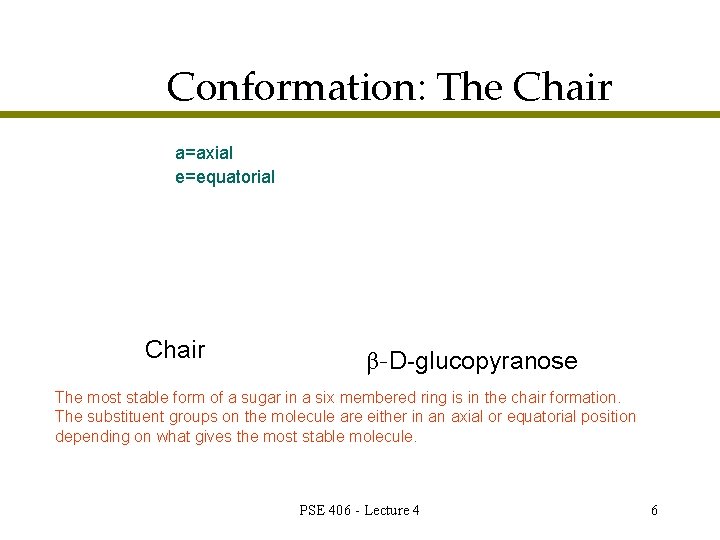
Conformation: The Chair a=axial e=equatorial Chair β-D-glucopyranose The most stable form of a sugar in a six membered ring is in the chair formation. The substituent groups on the molecule are either in an axial or equatorial position depending on what gives the most stable molecule. PSE 406 - Lecture 4 6

Conformation: Other Forms We won’t be worrying about these forms. PSE 406 - Lecture 4 7

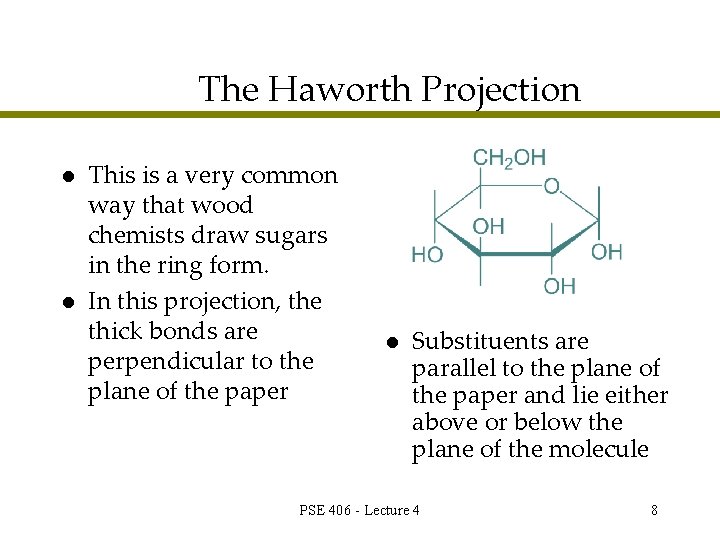
The Haworth Projection l l This is a very common way that wood chemists draw sugars in the ring form. In this projection, the thick bonds are perpendicular to the plane of the paper l Substituents are parallel to the plane of the paper and lie either above or below the plane of the molecule PSE 406 - Lecture 4 8

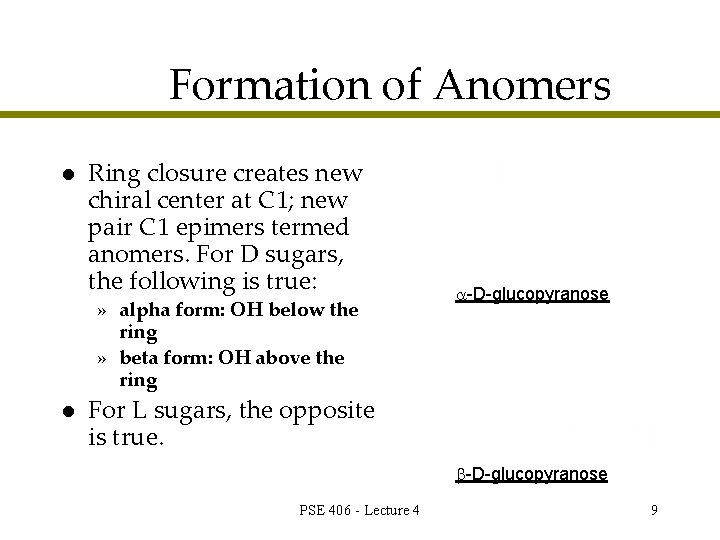
Formation of Anomers l Ring closure creates new chiral center at C 1; new pair C 1 epimers termed anomers. For D sugars, the following is true: » alpha form: OH below the ring » beta form: OH above the ring l -D-glucopyranose For L sugars, the opposite is true. -D-glucopyranose PSE 406 - Lecture 4 9

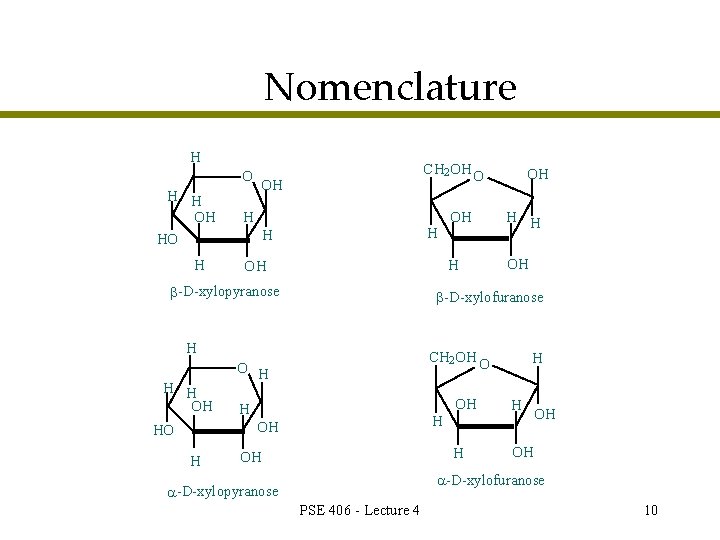
Nomenclature H O H H OH HO H CH 2 OH O OH H OH -D-xylopyranose H H OH CH 2 OH O O H H OH HO H H -D-xylofuranose H H H OH OH -D-xylofuranose -D-xylopyranose PSE 406 - Lecture 4 10

Mutarotation (1) Notes PSE 406 - Lecture 4 11

Mutarotation (2) l Note: the cyclic form must contain a hemi-form in order to have a migratory hydrogen and therefore be capable of ring-opening (that is, mutarotation). PSE 406 - Lecture 4 12

Fischer to Haworth l l In order to convert a Fischer projection of a sugar to a Haworth project, certain rules must be followed in order to maintain correct orientation of the molecule. Remember, the horizontal bonds in the Fischer projection (solid wedges) are out of the plane and the vertical wedges (broken wedges) are into the plane. PSE 406 - Lecture 4 13

Fischer to Haworth l l Modify the Fischer formula so that all of the ring atoms lie along a vertic Proceed around the Haworth formula placing the groups on the left abo PSE 406 - Lecture 4 14

Monosaccharides D Versus L, Part II PSE 406 - Lecture 4 15

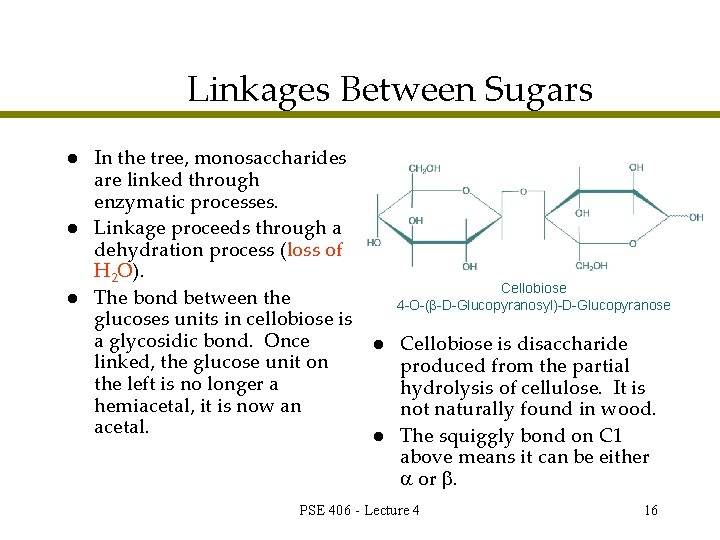
Linkages Between Sugars l l l In the tree, monosaccharides are linked through enzymatic processes. Linkage proceeds through a dehydration process (loss of H 2 O). The bond between the glucoses units in cellobiose is a glycosidic bond. Once linked, the glucose unit on the left is no longer a hemiacetal, it is now an acetal. Cellobiose 4 -O-( -D-Glucopyranosyl)-D-Glucopyranose l l Cellobiose is disaccharide produced from the partial hydrolysis of cellulose. It is not naturally found in wood. The squiggly bond on C 1 above means it can be either or . PSE 406 - Lecture 4 16

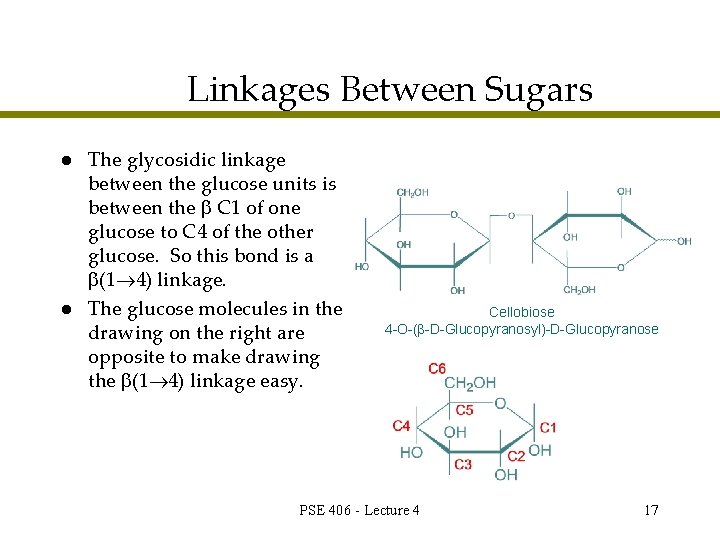
Linkages Between Sugars l l The glycosidic linkage between the glucose units is between the C 1 of one glucose to C 4 of the other glucose. So this bond is a (1 4) linkage. The glucose molecules in the drawing on the right are opposite to make drawing the (1 4) linkage easy. Cellobiose 4 -O-( -D-Glucopyranosyl)-D-Glucopyranose PSE 406 - Lecture 4 17

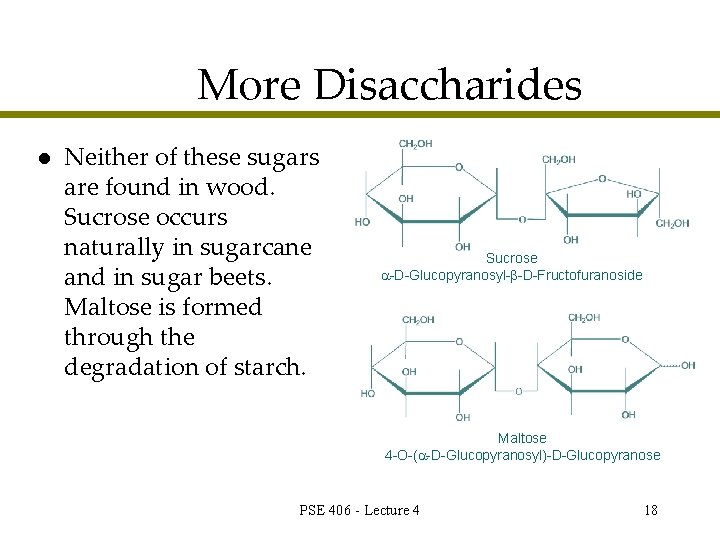
More Disaccharides l Neither of these sugars are found in wood. Sucrose occurs naturally in sugarcane and in sugar beets. Maltose is formed through the degradation of starch. Sucrose -D-Glucopyranosyl- -D-Fructofuranoside Maltose 4 -O-( -D-Glucopyranosyl)-D-Glucopyranose PSE 406 - Lecture 4 18

Reducing End Groups l l l Sugars containing aldehydes and ketones are referred to as reducing sugars. These sugars will reduce copper in a specific reducing substances test. The aldehyde or ketone groups are oxidized by the copper. Sucrose is not a reducing substance Fehling’s Reaction: deep blue solution to red orange precipitate. PSE 406 - Lecture 4 19
 More more more i want more more more more we praise you
More more more i want more more more more we praise you More more more i want more more more more we praise you
More more more i want more more more more we praise you Glucose seliwanoff test
Glucose seliwanoff test Pse wood meaning
Pse wood meaning Pse wood meaning
Pse wood meaning Softwood anatomy
Softwood anatomy Roots method of vegetative propagation
Roots method of vegetative propagation Esharenet
Esharenet Esau wood poem
Esau wood poem Wood wood teenager
Wood wood teenager Bulk flow in phloem
Bulk flow in phloem Aldo sugars
Aldo sugars Glucose standard curve
Glucose standard curve Qualitative analysis of carbohydrate
Qualitative analysis of carbohydrate Basic monosaccharides
Basic monosaccharides Sucrose fehling's test
Sucrose fehling's test Reducing vs non reducing sugars
Reducing vs non reducing sugars Sugars aurora
Sugars aurora Alan sugar's middle name
Alan sugar's middle name Mrs couzens
Mrs couzens




































