Wood Chemistry PSE 406 Lecture 5 Cellulose PSE


- Slides: 21

Wood Chemistry PSE 406 Lecture 5 Cellulose PSE 406: Lecture 5 1

Ketoses l α-D-fructopyranose l β-D-fructofuranose PSE 406: Lecture 5 2

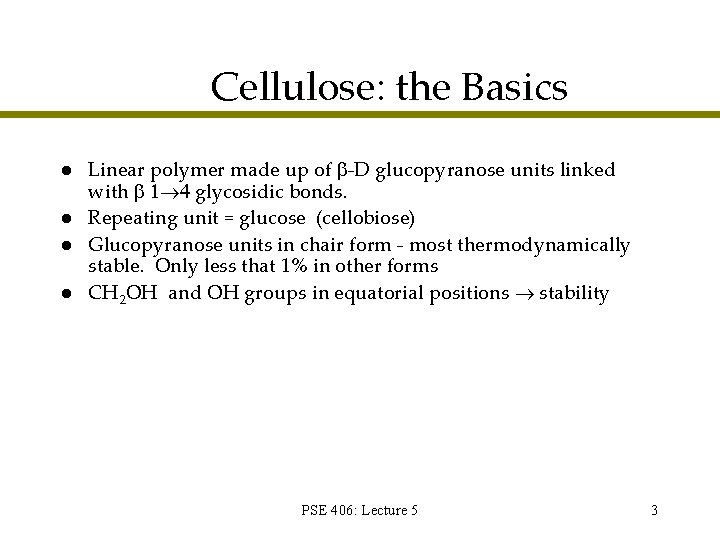
Cellulose: the Basics l l Linear polymer made up of -D glucopyranose units linked with glycosidic bonds. Repeating unit = glucose (cellobiose) Glucopyranose units in chair form - most thermodynamically stable. Only less that 1% in other forms CH 2 OH and OH groups in equatorial positions stability PSE 406: Lecture 5 3

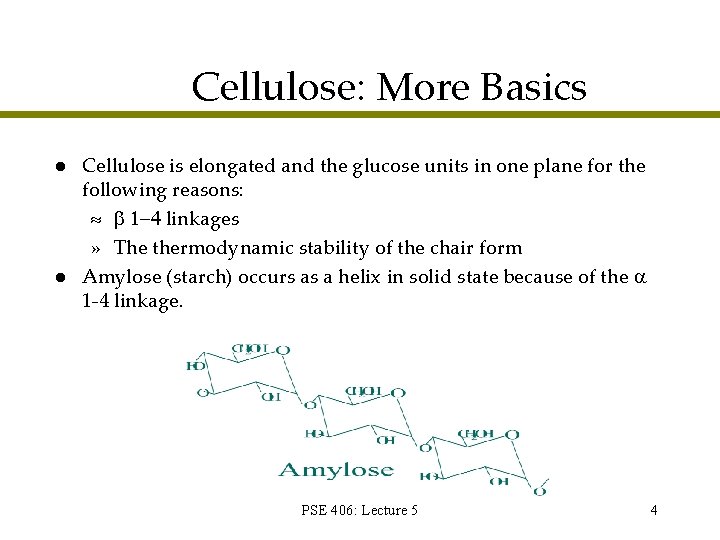
Cellulose: More Basics l l Cellulose is elongated and the glucose units in one plane for the following reasons: » linkages » The thermodynamic stability of the chair form Amylose (starch) occurs as a helix in solid state because of the 1 -4 linkage. PSE 406: Lecture 5 4

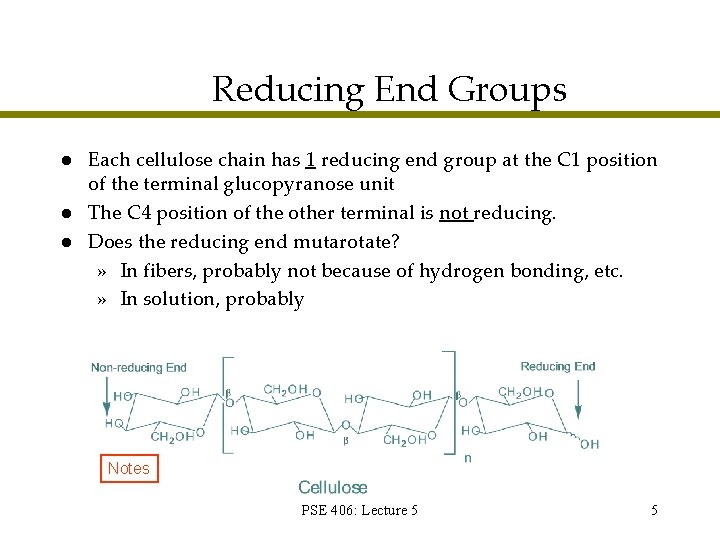
Reducing End Groups l l l Each cellulose chain has 1 reducing end group at the C 1 position of the terminal glucopyranose unit The C 4 position of the other terminal is not reducing. Does the reducing end mutarotate? » In fibers, probably not because of hydrogen bonding, etc. » In solution, probably Notes PSE 406: Lecture 5 5

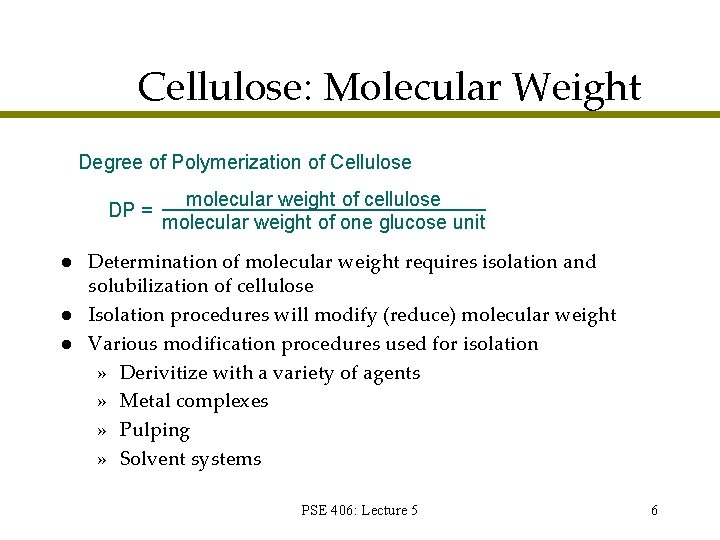
Cellulose: Molecular Weight Degree of Polymerization of Cellulose DP = l l l molecular weight of cellulose molecular weight of one glucose unit Determination of molecular weight requires isolation and solubilization of cellulose Isolation procedures will modify (reduce) molecular weight Various modification procedures used for isolation » Derivitize with a variety of agents » Metal complexes » Pulping » Solvent systems PSE 406: Lecture 5 6

Degree of Polymerization Notes PSE 406: Lecture 5 7

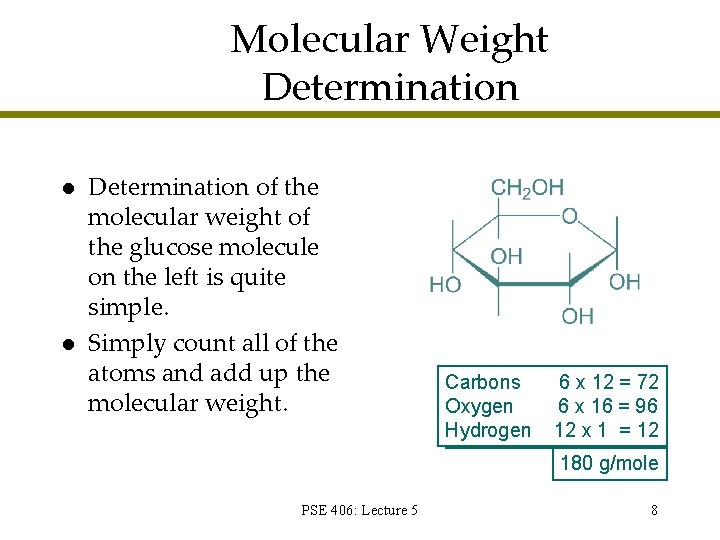
Molecular Weight Determination l l Determination of the molecular weight of the glucose molecule on the left is quite simple. Simply count all of the atoms and add up the molecular weight. Carbons Oxygen Hydrogen 6 x 12 = 72 6 x 16 = 96 12 x 1 = 12 180 g/mole PSE 406: Lecture 5 8

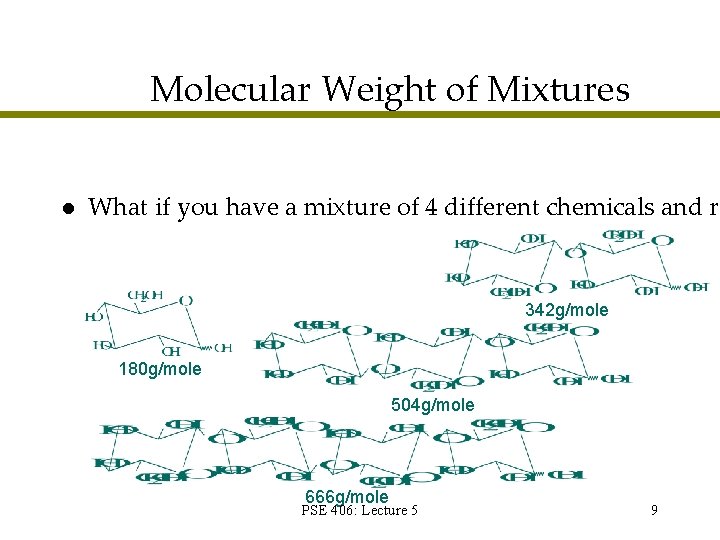
Molecular Weight of Mixtures l What if you have a mixture of 4 different chemicals and re 342 g/mole 180 g/mole 504 g/mole 666 g/mole PSE 406: Lecture 5 9

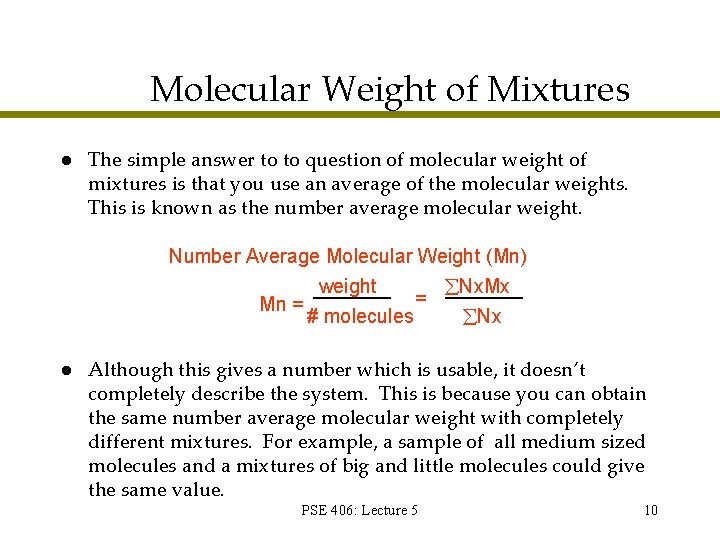
Molecular Weight of Mixtures l The simple answer to to question of molecular weight of mixtures is that you use an average of the molecular weights. This is known as the number average molecular weight. Number Average Molecular Weight (Mn) Mn = l weight # molecules = Nx. Mx Nx Although this gives a number which is usable, it doesn’t completely describe the system. This is because you can obtain the same number average molecular weight with completely different mixtures. For example, a sample of all medium sized molecules and a mixtures of big and little molecules could give the same value. PSE 406: Lecture 5 10

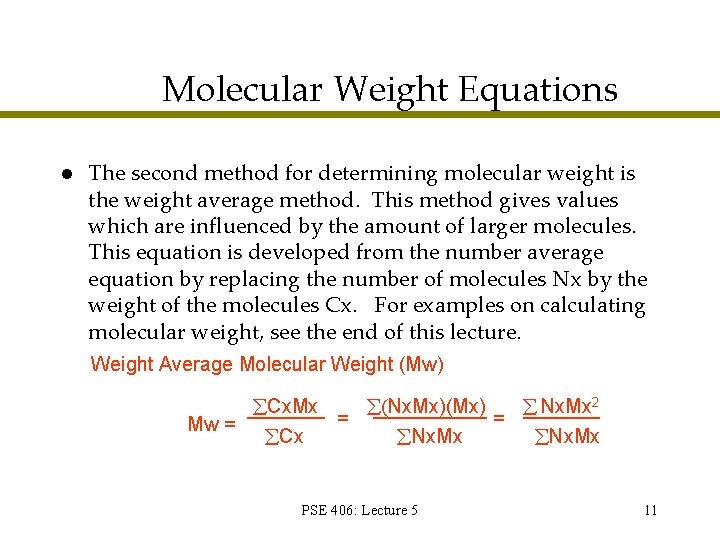
Molecular Weight Equations l The second method for determining molecular weight is the weight average method. This method gives values which are influenced by the amount of larger molecules. This equation is developed from the number average equation by replacing the number of molecules Nx by the weight of the molecules Cx. For examples on calculating molecular weight, see the end of this lecture. Weight Average Molecular Weight (Mw) Cx. Mx Nx. Mx)(Mx) Nx. Mx 2 = = Mw = Cx Nx. Mx PSE 406: Lecture 5 11

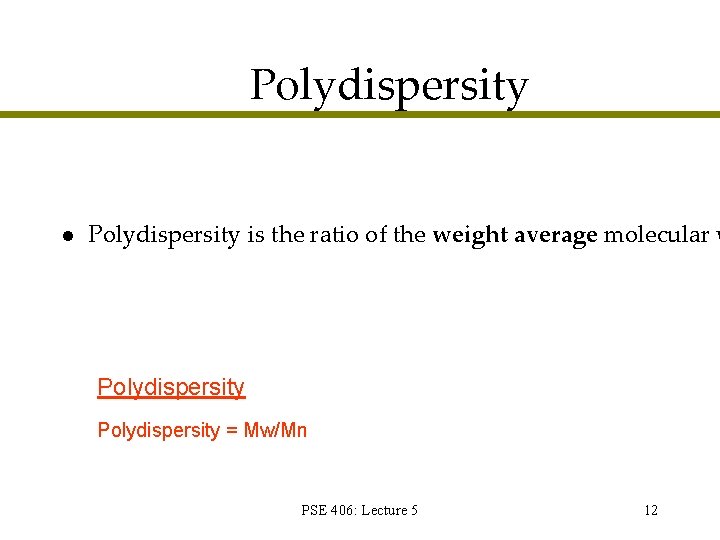
Polydispersity l Polydispersity is the ratio of the weight average molecular w Polydispersity = Mw/Mn PSE 406: Lecture 5 12

Lecture 5 Example Problems PSE 406: Lecture 5 13

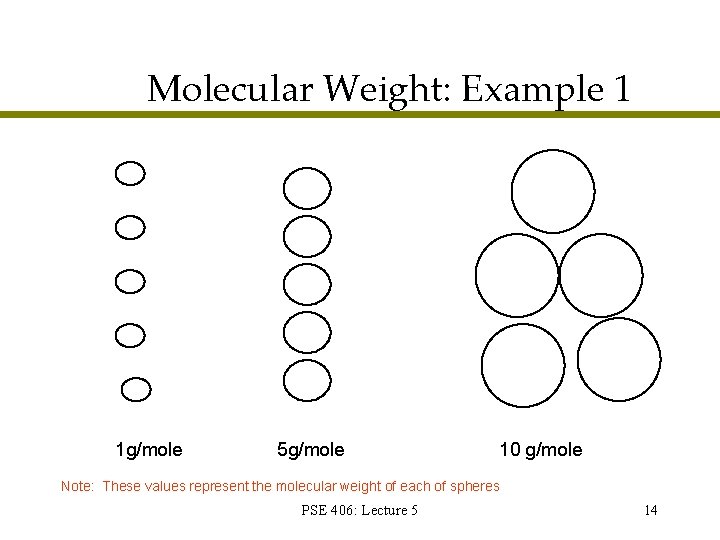
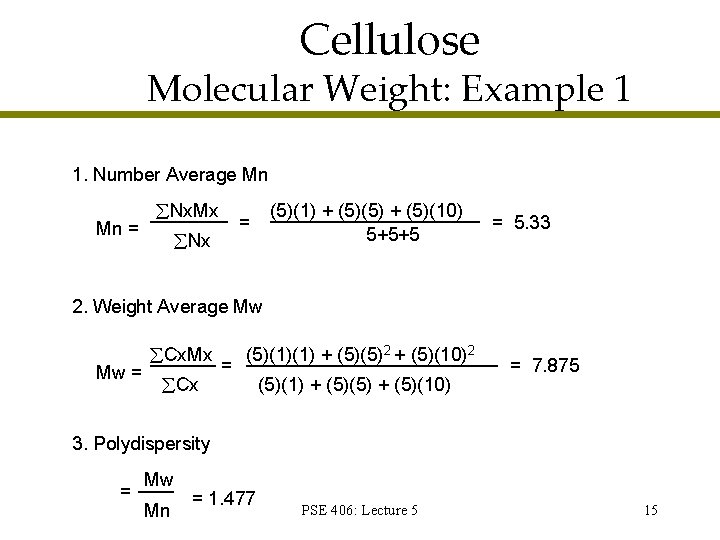
Molecular Weight: Example 1 1 g/mole 5 g/mole 10 g/mole Note: These values represent the molecular weight of each of spheres PSE 406: Lecture 5 14

Cellulose Molecular Weight: Example 1 1. Number Average Mn Mn = Nx. Mx (5)(1) + (5)(5) + (5)(10) 5+5+5 = Nx = 5. 33 2. Weight Average Mw Mw = Cx. Mx Cx = (5)(1)(1) + (5)(5)2 + (5)(10)2 (5)(1) + (5)(5) + (5)(10) = 7. 875 3. Polydispersity = Mw Mn = 1. 477 PSE 406: Lecture 5 15

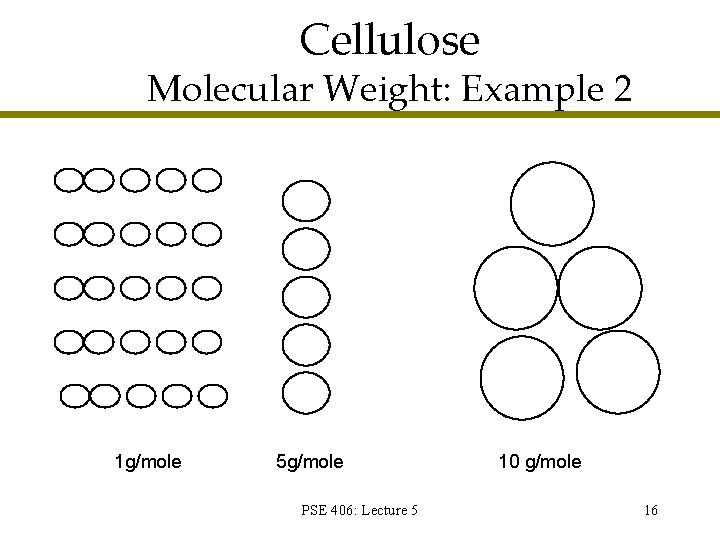
Cellulose Molecular Weight: Example 2 1 g/mole 5 g/mole PSE 406: Lecture 5 10 g/mole 16

Cellulose Molecular Weight: Example 2 PSE 406: Lecture 5 17

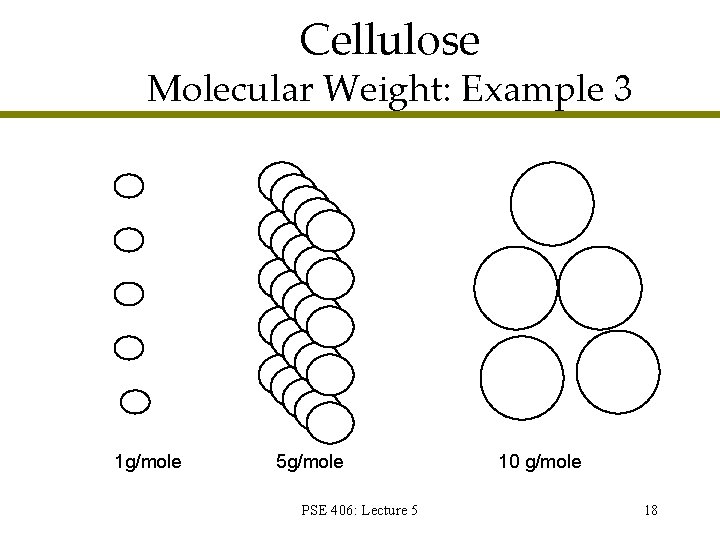
Cellulose Molecular Weight: Example 3 1 g/mole 5 g/mole PSE 406: Lecture 5 10 g/mole 18

Cellulose Molecular Weight: Example 3 PSE 406: Lecture 5 19

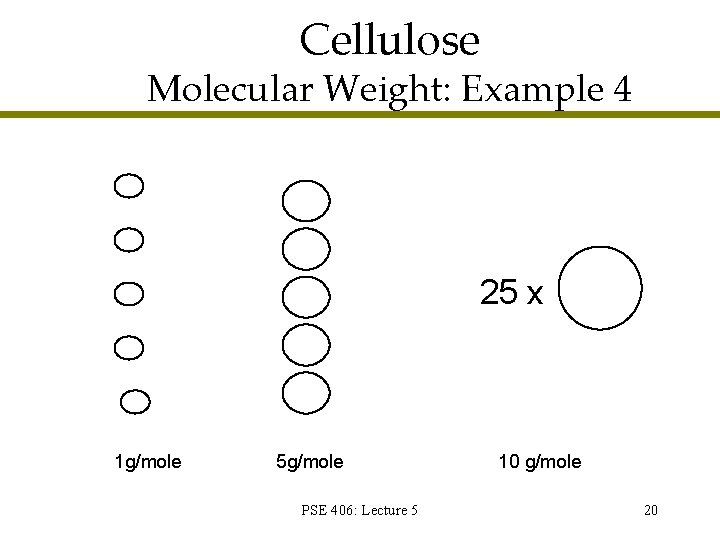
Cellulose Molecular Weight: Example 4 25 x 1 g/mole 5 g/mole PSE 406: Lecture 5 10 g/mole 20

Cellulose Molecular Weight: Example 4 PSE 406: Lecture 5 21