What is Light 1 Waves z Wavelength length









![Periodic Patterns z Example - Germanium [Ar] 2 4 s 10 3 d 2 Periodic Patterns z Example - Germanium [Ar] 2 4 s 10 3 d 2](https://slidetodoc.com/presentation_image_h2/8476e8d607eedb58cabbf5ff5c3dd5b0/image-40.jpg)
![Stability z Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d Stability z Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/8476e8d607eedb58cabbf5ff5c3dd5b0/image-42.jpg)
![Stability z Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d Stability z Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/8476e8d607eedb58cabbf5ff5c3dd5b0/image-43.jpg)

- Slides: 46

What is Light? 1

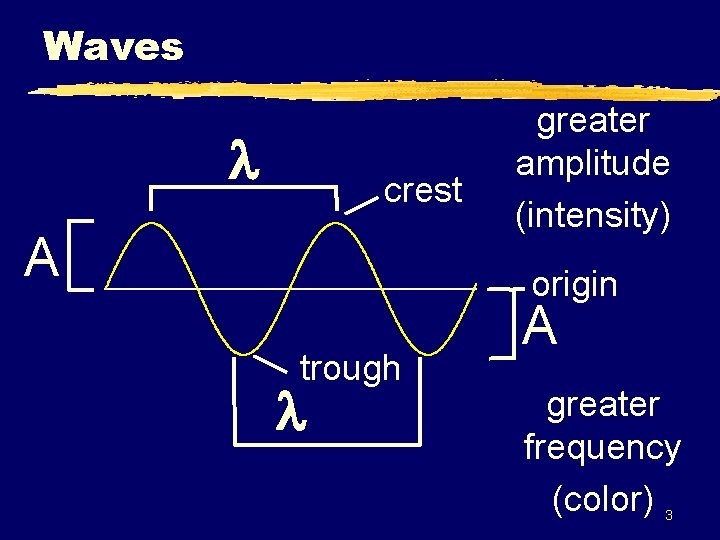
Waves z Wavelength ( ) - length of one complete wave z Frequency ( ) - # of waves that pass a point during a certain time period y hertz (Hz) = 1/s z Amplitude (A) - distance from the origin to the trough or crest 2

Waves crest A greater amplitude (intensity) origin trough A greater frequency (color) 3

EM Spectrum H I G H L O W E N E R G Y 4

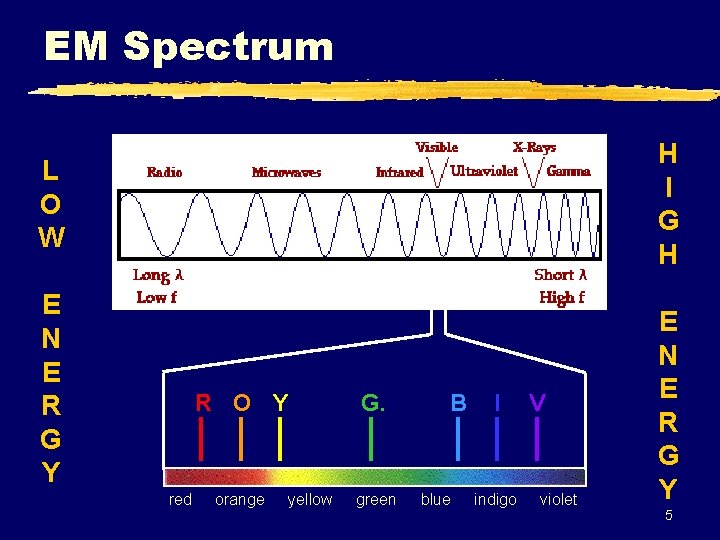
EM Spectrum H I G H L O W E N E R G Y red R O Y G. orange green yellow B blue I indigo V violet E N E R G Y 5

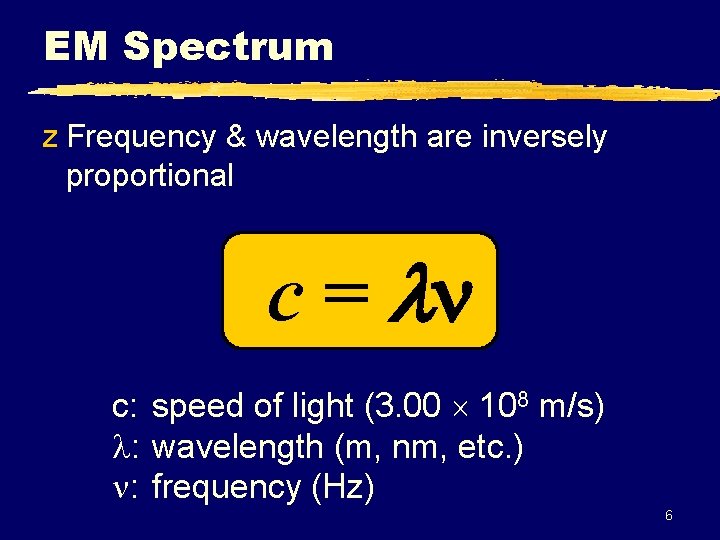
EM Spectrum z Frequency & wavelength are inversely proportional c = c: speed of light (3. 00 108 m/s) : wavelength (m, nm, etc. ) : frequency (Hz) 6

EM Spectrum z EX: Find the frequency of a photon with a wavelength of 434 nm. GIVEN: WORK: =c =? = 434 nm = 4. 34 10 -7 m = 3. 00 108 m/s -7 m 8 4. 34 10 c = 3. 00 10 m/s = 6. 91 1014 Hz 7

Light is more than waves… z Planck (1900) y Observed - emission of light from hot objects y Concluded - energy is emitted in small, specific amounts (quanta) 8

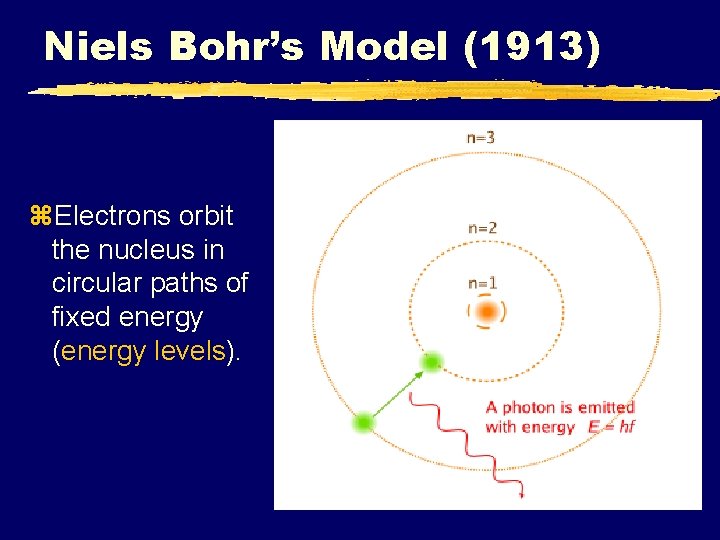
Niels Bohr’s Model (1913) z. Electrons orbit the nucleus in circular paths of fixed energy (energy levels).

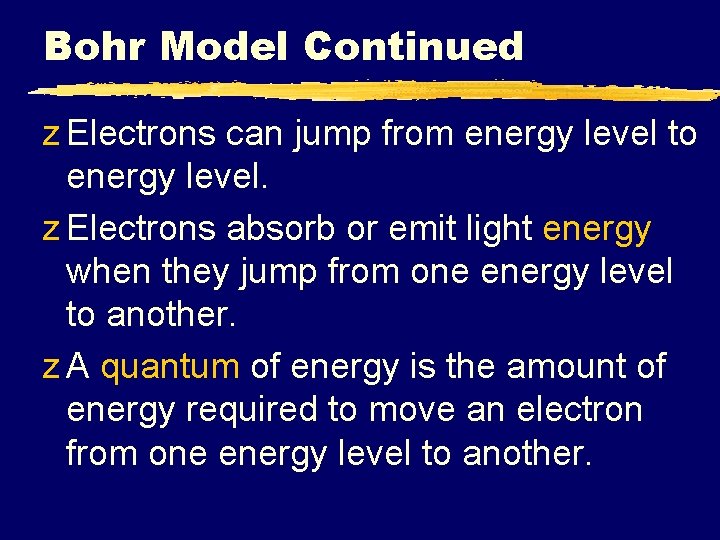
Bohr Model Continued z Electrons can jump from energy level to energy level. z Electrons absorb or emit light energy when they jump from one energy level to another. z A quantum of energy is the amount of energy required to move an electron from one energy level to another.

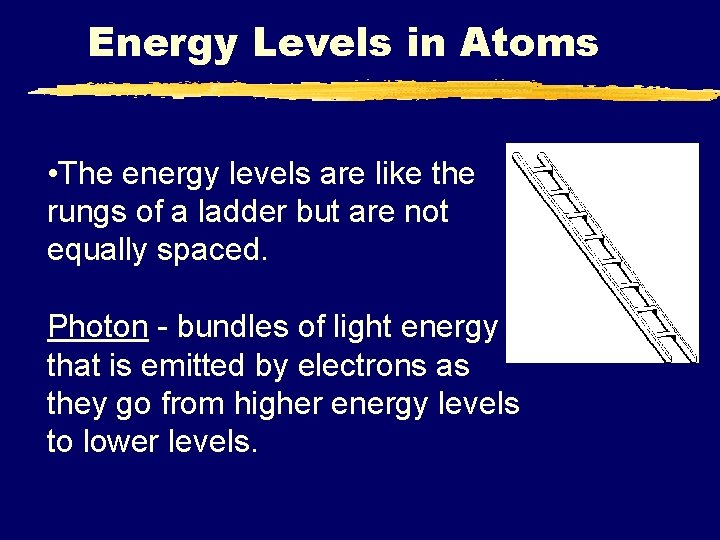
Energy Levels in Atoms • The energy levels are like the rungs of a ladder but are not equally spaced. Photon - bundles of light energy that is emitted by electrons as they go from higher energy levels to lower levels.

Photons z Einstein (1905) y Concluded - light has properties of both waves and particles “wave-particle duality” 12

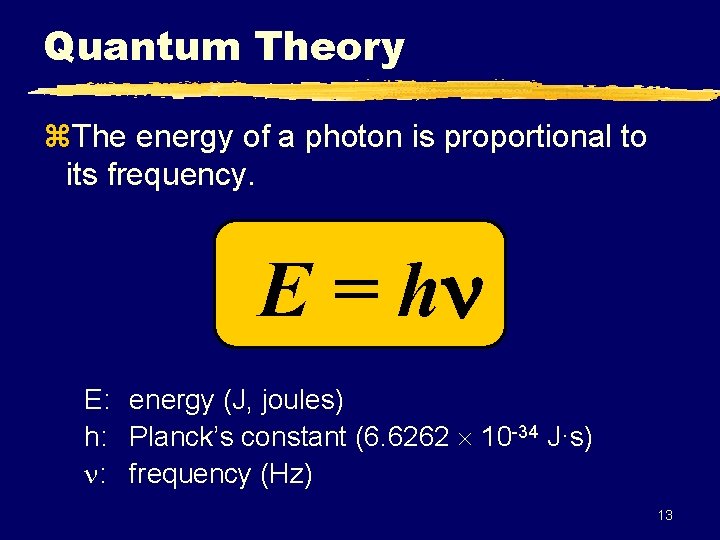
Quantum Theory z. The energy of a photon is proportional to its frequency. E = h E: energy (J, joules) h: Planck’s constant (6. 6262 10 -34 J·s) : frequency (Hz) 13

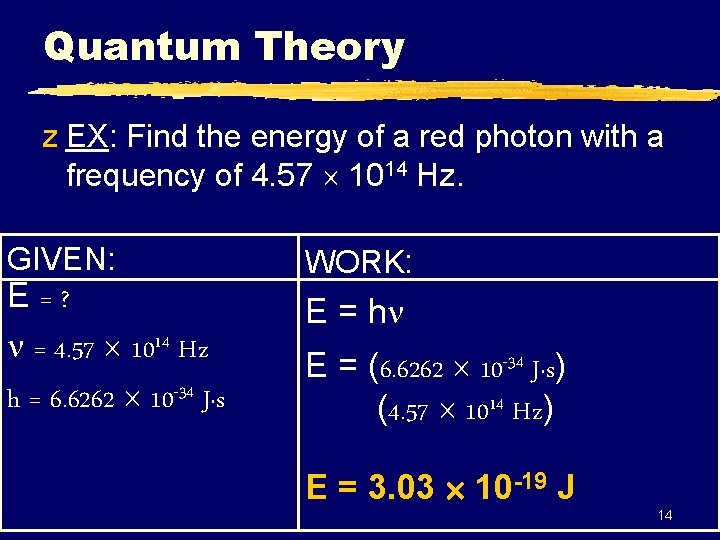
Quantum Theory z EX: Find the energy of a red photon with a frequency of 4. 57 1014 Hz. GIVEN: E=? = 4. 57 1014 Hz h = 6. 6262 10 -34 J·s WORK: E = h E = (6. 6262 10 -34 J·s) (4. 57 1014 Hz) E = 3. 03 10 -19 J 14

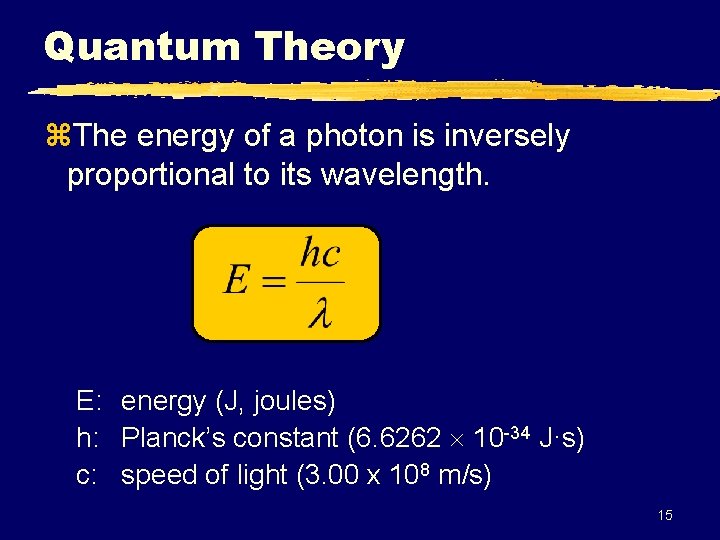
Quantum Theory z. The energy of a photon is inversely proportional to its wavelength. E: energy (J, joules) h: Planck’s constant (6. 6262 10 -34 J·s) c: speed of light (3. 00 x 108 m/s) 15

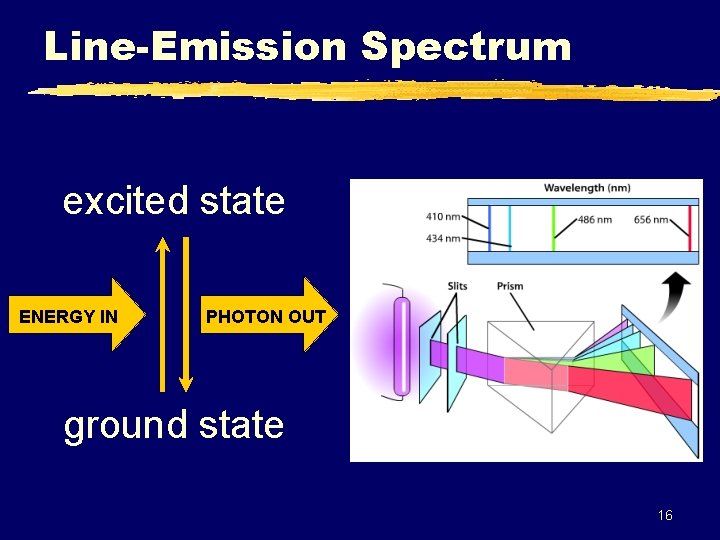
Line-Emission Spectrum excited state ENERGY IN PHOTON OUT ground state 16

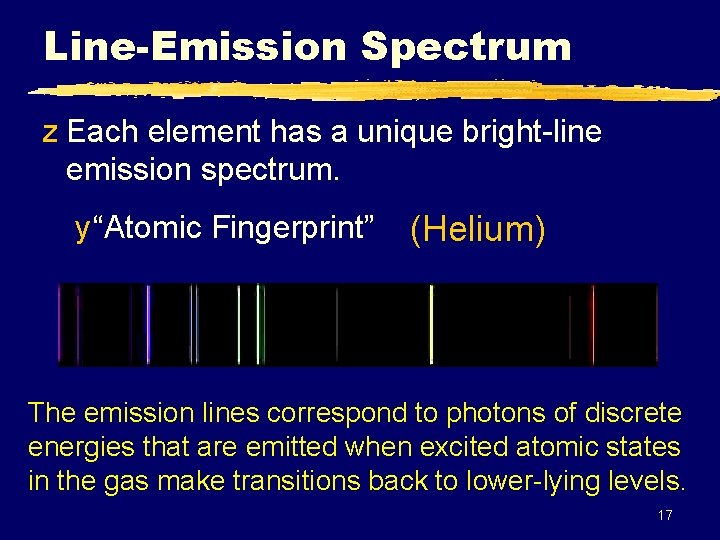
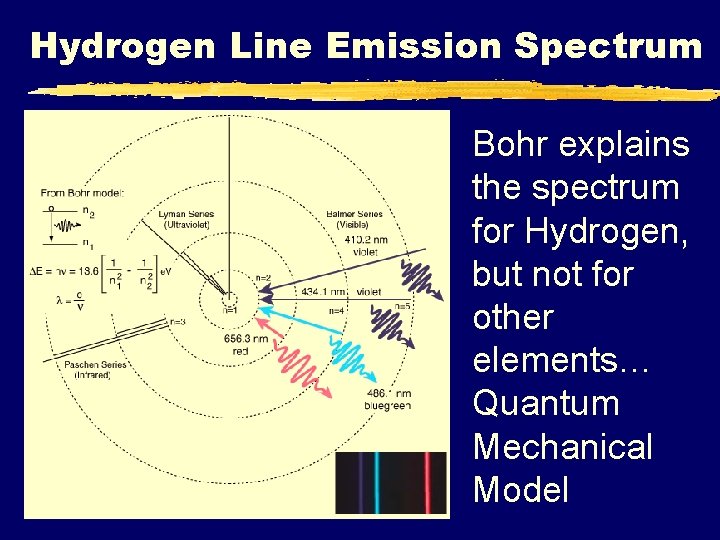
Line-Emission Spectrum z Each element has a unique bright-line emission spectrum. y “Atomic Fingerprint” (Helium) The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels. 17

Hydrogen Line Emission Spectrum Bohr explains the spectrum for Hydrogen, but not for other elements… Quantum Mechanical Model

Part 2: Quantum Mechanics 19

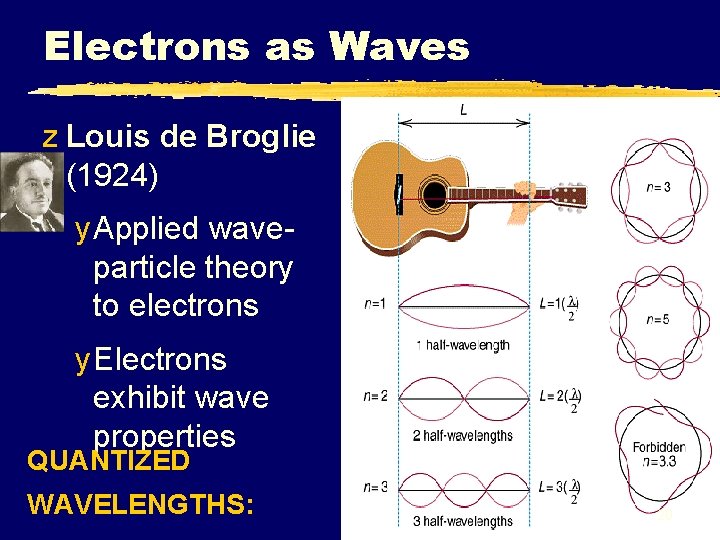
Electrons as Waves z Louis de Broglie (1924) y Applied waveparticle theory to electrons y Electrons exhibit wave properties QUANTIZED WAVELENGTHS: 20

Quantum Mechanics z Schrödinger and “wavefunction” y The wavefunction helps describes the location of the electron, and its probable location 21

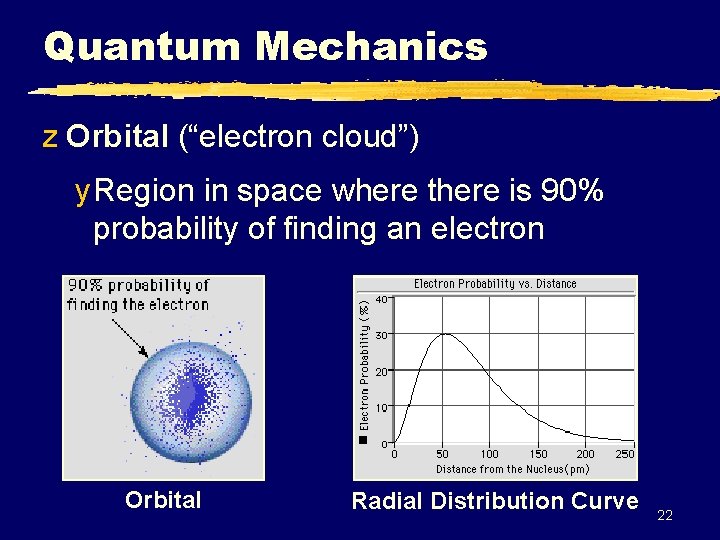
Quantum Mechanics z Orbital (“electron cloud”) y Region in space where there is 90% probability of finding an electron Orbital Radial Distribution Curve 22

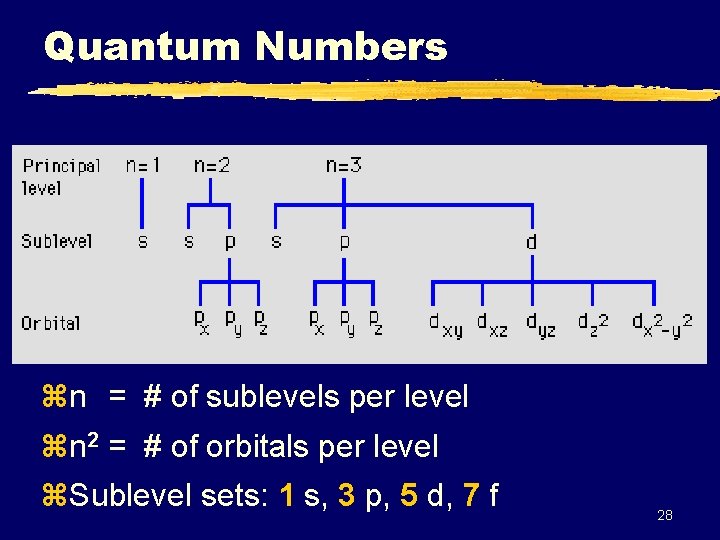
Quantum Mechanics z Quantum Numbers: y Specify the “address” of each electron in an atom 23

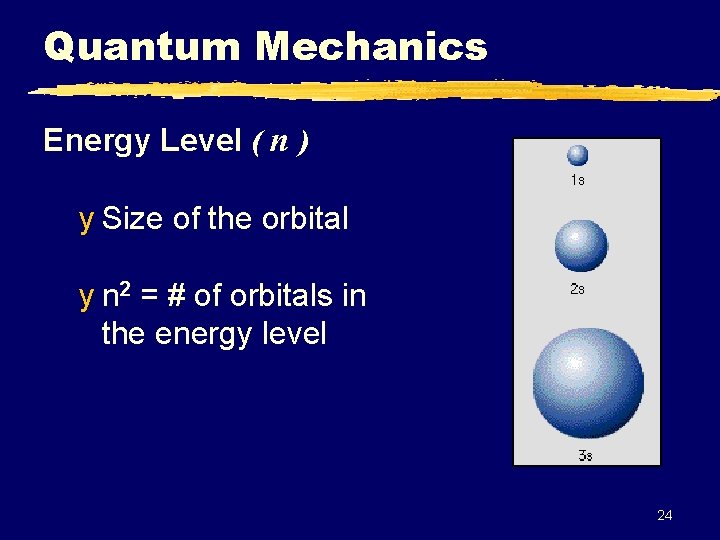
Quantum Mechanics Energy Level ( n ) y Size of the orbital y n 2 = # of orbitals in the energy level 24

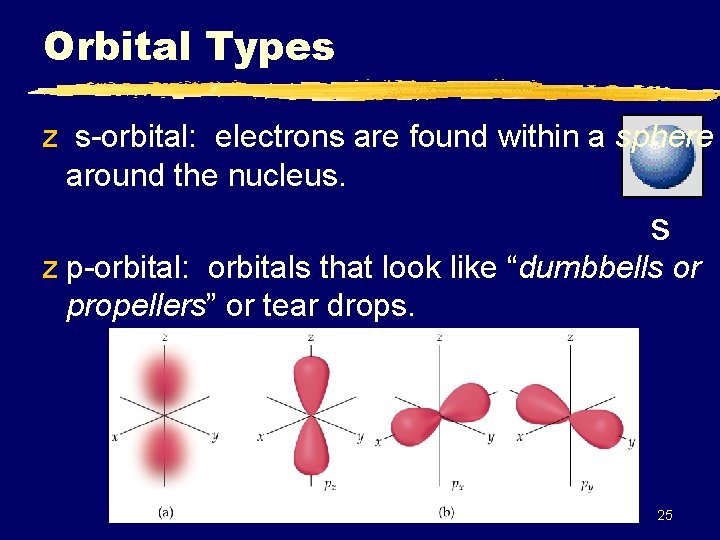
Orbital Types z s-orbital: electrons are found within a sphere around the nucleus. s z p-orbital: orbitals that look like “dumbbells or propellers” or tear drops. 25

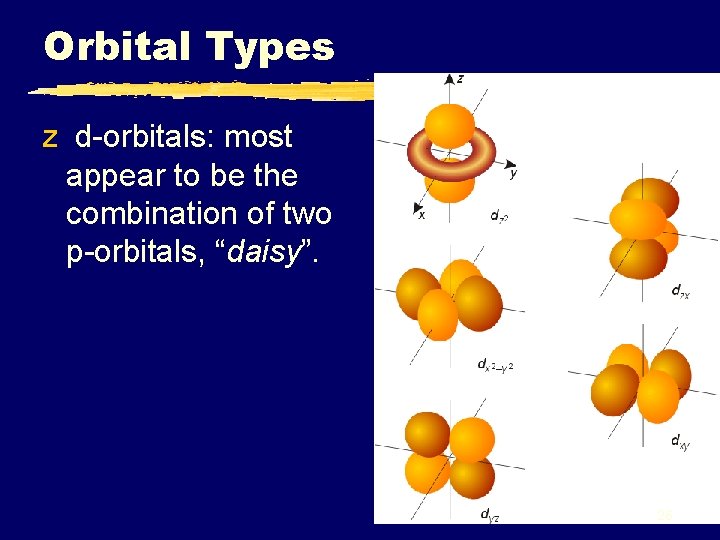
Orbital Types z d-orbitals: most appear to be the combination of two p-orbitals, “daisy”. 26

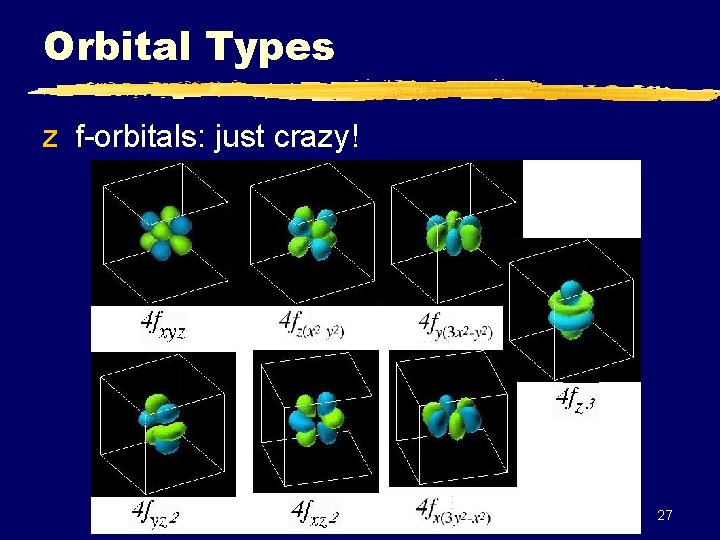
Orbital Types z f-orbitals: just crazy! 27

Quantum Numbers zn = # of sublevels per level zn 2 = # of orbitals per level z. Sublevel sets: 1 s, 3 p, 5 d, 7 f 28

Quantum Numbers y. Atom is the filing cabinet y. Each drawer is an energy level y. Folders are sublevels (s, p, d, etc. ) y. Papers in the folder are orbitals 29

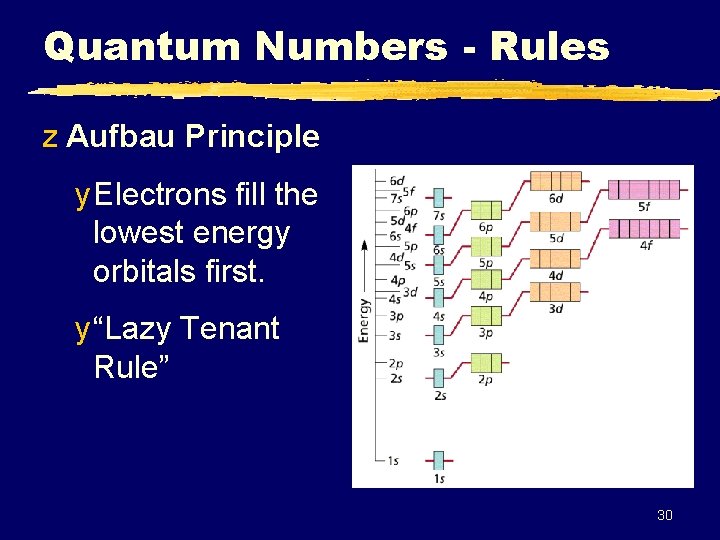
Quantum Numbers - Rules z Aufbau Principle y Electrons fill the lowest energy orbitals first. y “Lazy Tenant Rule” 30

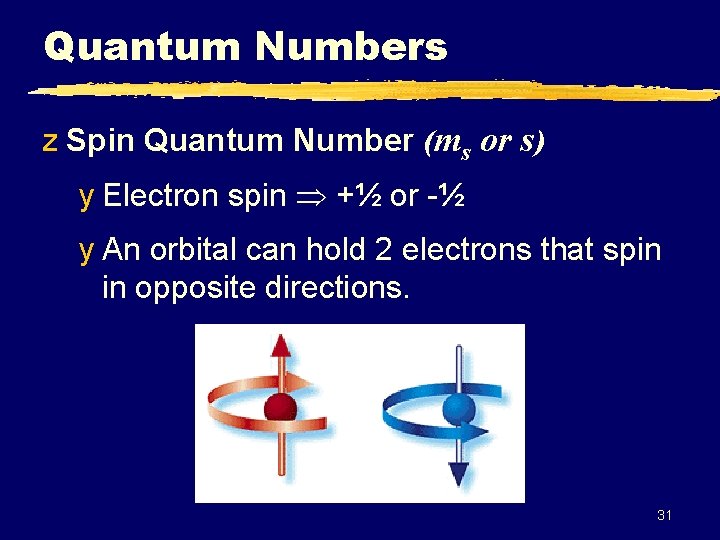
Quantum Numbers z Spin Quantum Number (ms or s) y Electron spin +½ or -½ y An orbital can hold 2 electrons that spin in opposite directions. 31

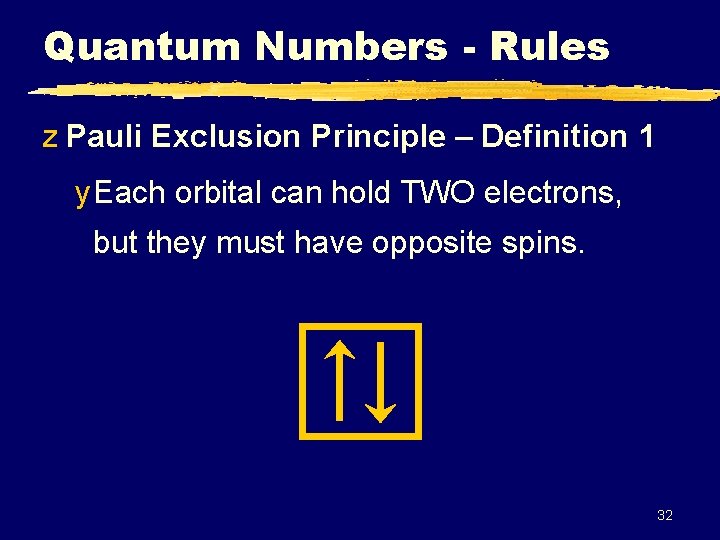
Quantum Numbers - Rules z Pauli Exclusion Principle – Definition 1 y Each orbital can hold TWO electrons, but they must have opposite spins. 32

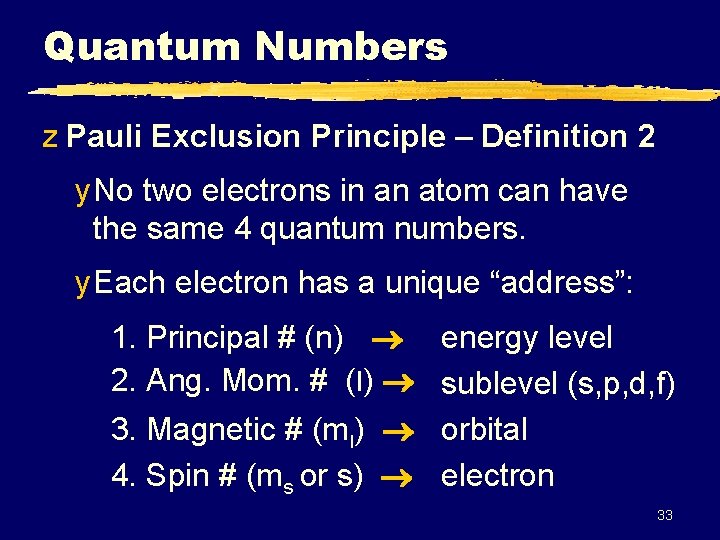
Quantum Numbers z Pauli Exclusion Principle – Definition 2 y No two electrons in an atom can have the same 4 quantum numbers. y Each electron has a unique “address”: 1. Principal # (n) energy level 2. Ang. Mom. # (l) sublevel (s, p, d, f) 3. Magnetic # (ml) orbital 4. Spin # (ms or s) electron 33

Quantum Numbers - Rules z Hund’s Rule y Within a sublevel, place one e- per orbital before pairing them. WRONG RIGHT 34

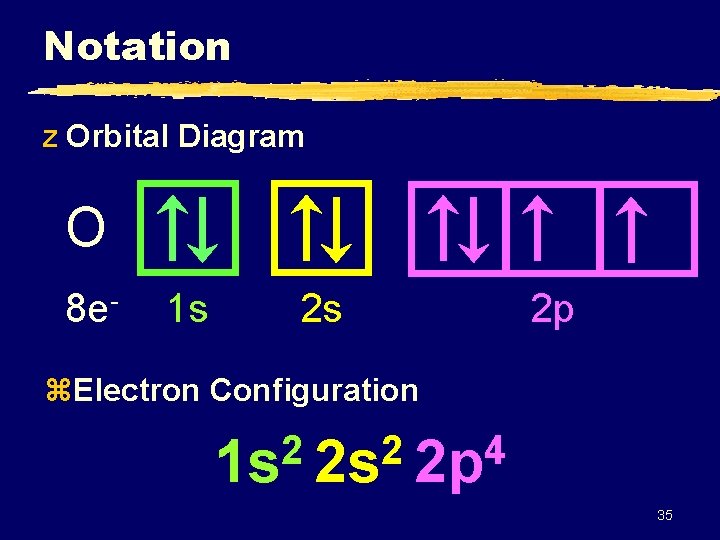
Notation z Orbital Diagram O 8 e- 1 s 2 s 2 p z. Electron Configuration 2 2 4 1 s 2 s 2 p 35

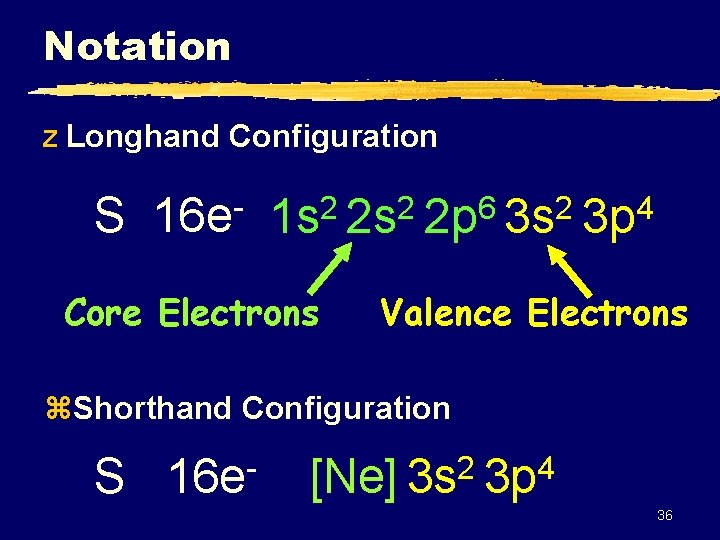
Notation z Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons 4 3 p Valence Electrons z. Shorthand Configuration S 16 e 2 4 [Ne] 3 s 3 p 36

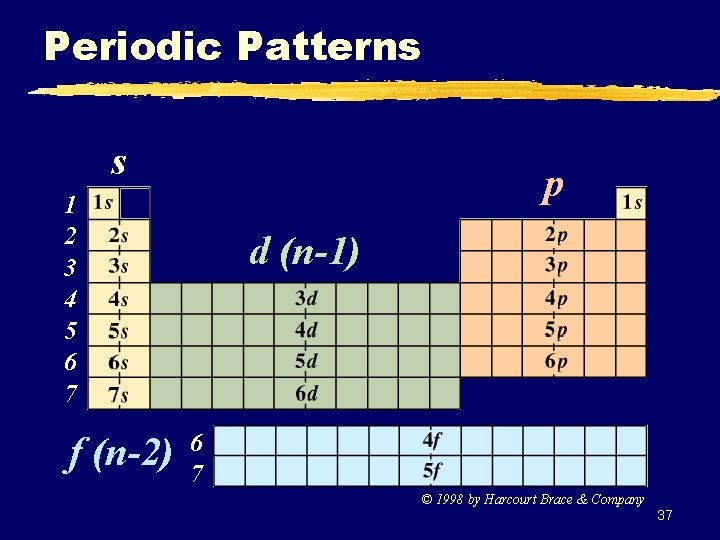
Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company 37

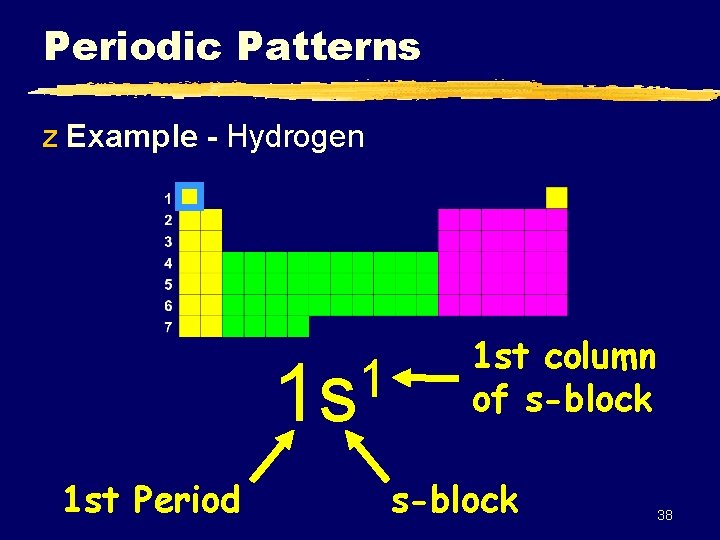
Periodic Patterns z Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block 38

Periodic Patterns z Shorthand Configuration y Core e-: Go up one row and over to the Noble Gas. y Valence e-: On the next row, fill in the # of e- in each sublevel. 39
![Periodic Patterns z Example Germanium Ar 2 4 s 10 3 d 2 Periodic Patterns z Example - Germanium [Ar] 2 4 s 10 3 d 2](https://slidetodoc.com/presentation_image_h2/8476e8d607eedb58cabbf5ff5c3dd5b0/image-40.jpg)
Periodic Patterns z Example - Germanium [Ar] 2 4 s 10 3 d 2 4 p 40

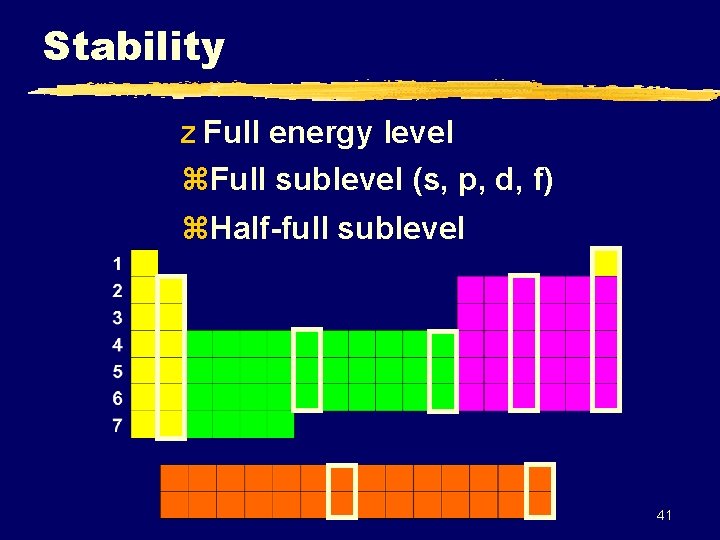
Stability z Full energy level z. Full sublevel (s, p, d, f) z. Half-full sublevel 41
![Stability z Electron Configuration Exceptions y Copper EXPECT Ar 4 s 2 3 d Stability z Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/8476e8d607eedb58cabbf5ff5c3dd5b0/image-42.jpg)
Stability z Electron Configuration Exceptions y. Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 y. Copper gains stability with a full d-sublevel. 42
![Stability z Electron Configuration Exceptions y Chromium EXPECT Ar 4 s 2 3 d Stability z Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d](https://slidetodoc.com/presentation_image_h2/8476e8d607eedb58cabbf5ff5c3dd5b0/image-43.jpg)
Stability z Electron Configuration Exceptions y. Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 y. Chromium gains stability with a half-full d-sublevel. 43

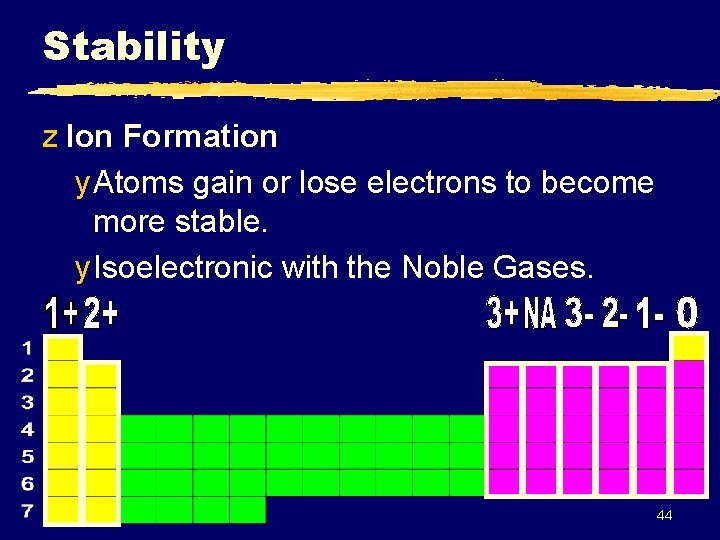
Stability z Ion Formation y Atoms gain or lose electrons to become more stable. y Isoelectronic with the Noble Gases. 44

Stability z Ion Electron Configuration y Write the e- config for the closest Noble Gas y EX: Oxygen ion O 2 - Ne 2 O 10 e [He] 2 2 s 6 2 p 45

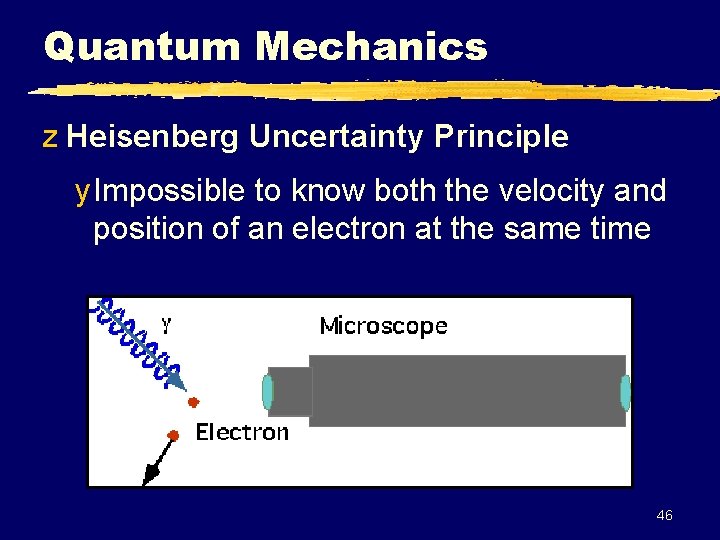
Quantum Mechanics z Heisenberg Uncertainty Principle y Impossible to know both the velocity and position of an electron at the same time 46