Waves and particles Ch 4 Waves Wavelength the










- Slides: 16

Waves and particles Ch. 4

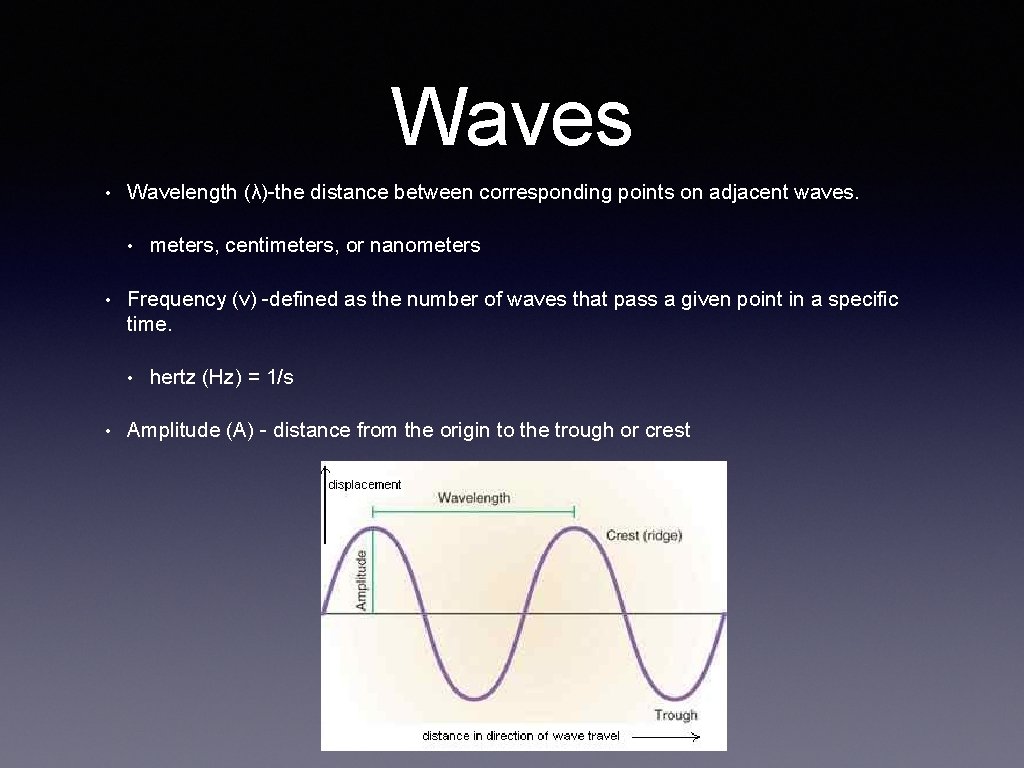
Waves • Wavelength (λ)-the distance between corresponding points on adjacent waves. • • Frequency (ν) -defined as the number of waves that pass a given point in a specific time. • • meters, centimeters, or nanometers hertz (Hz) = 1/s Amplitude (A) - distance from the origin to the trough or crest


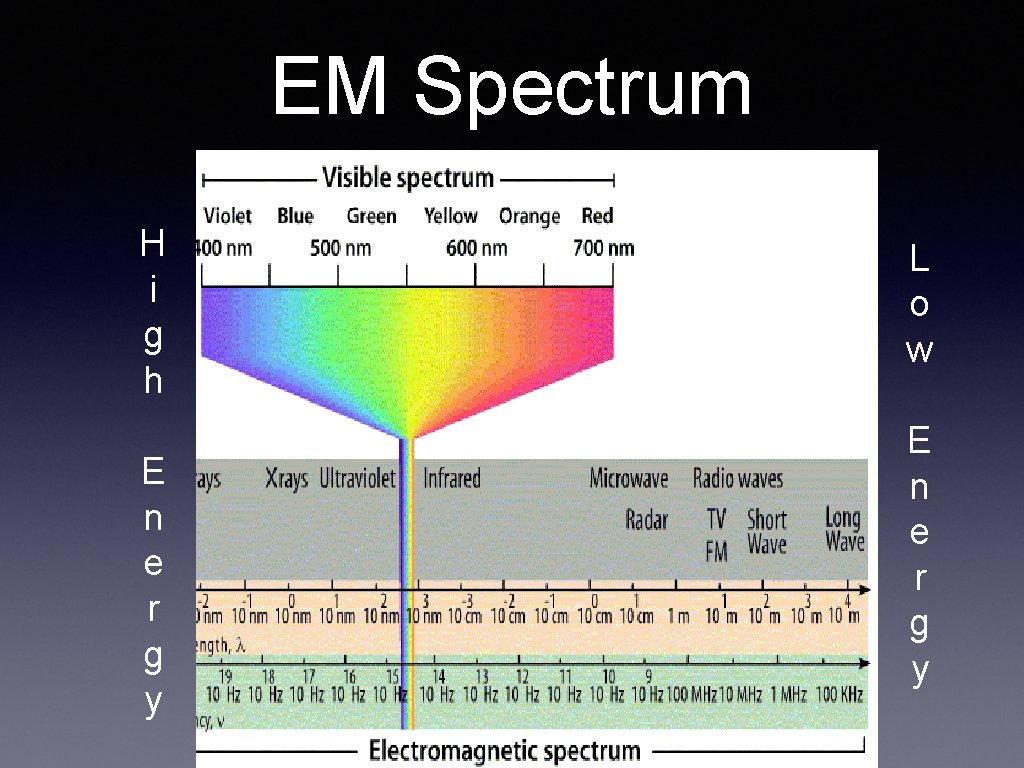
EM Spectrum H i g h E n e r g y L o w E n e r g y

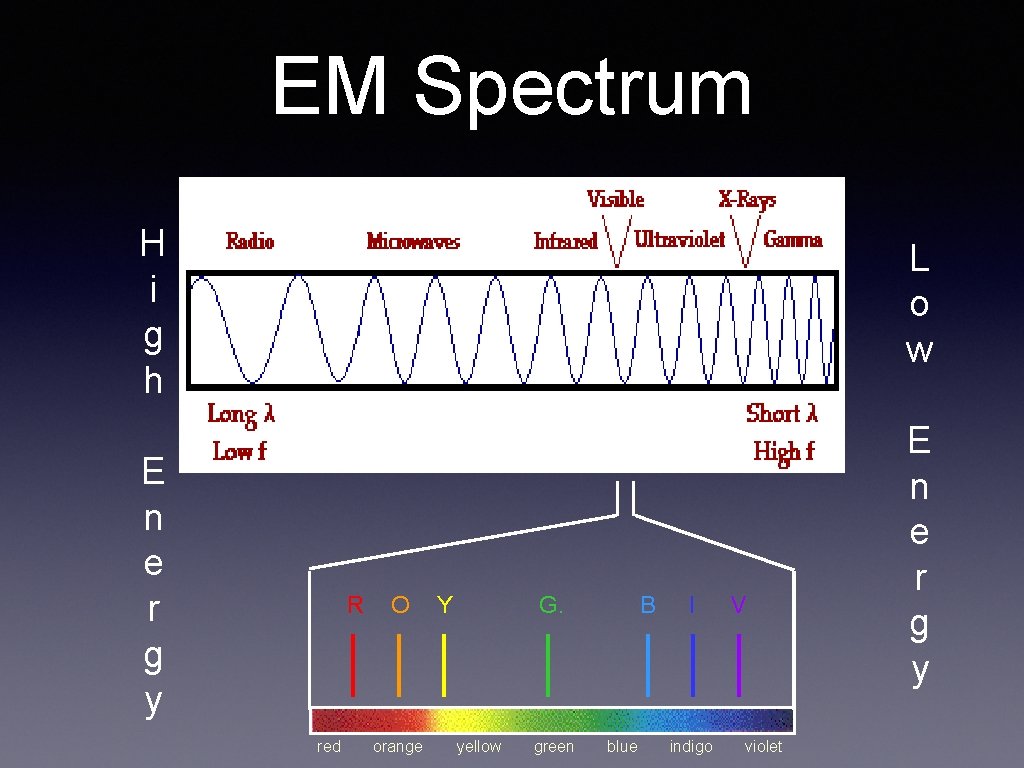
EM Spectrum H i g h L o w E n e r g y R red O orange Y G. yellow green B blue I indigo V violet E n e r g y

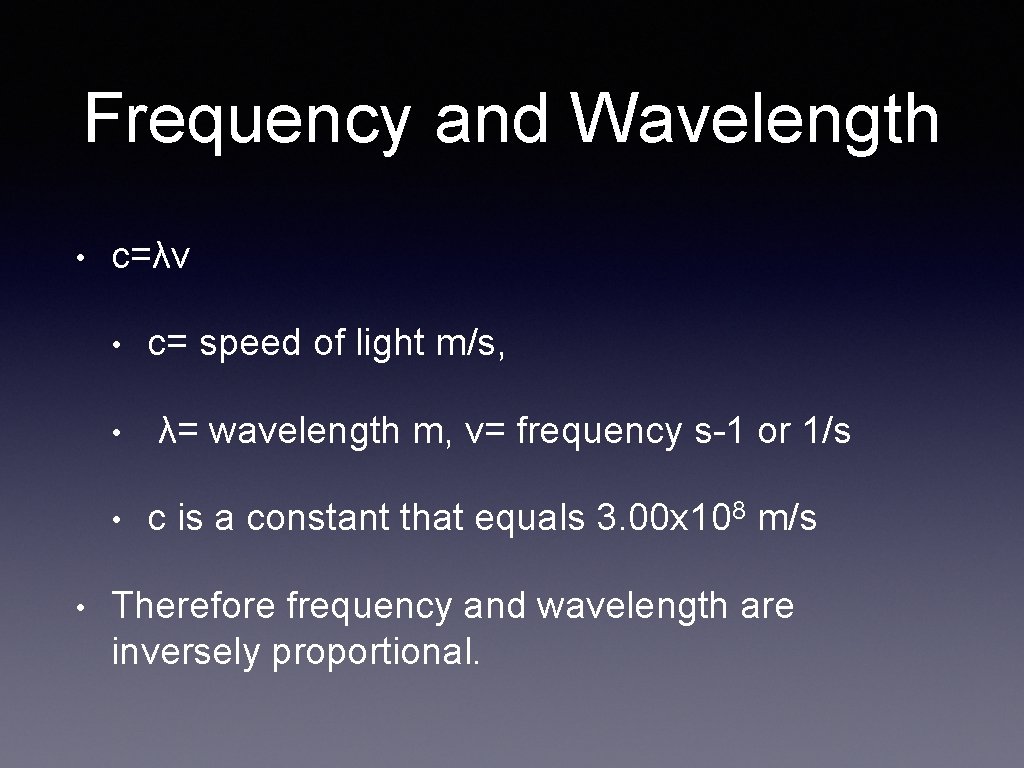
Frequency and Wavelength • c=λv • • c= speed of light m/s, λ= wavelength m, v= frequency s-1 or 1/s c is a constant that equals 3. 00 x 108 m/s Therefore frequency and wavelength are inversely proportional.

• Ex. Find the frequency of a photon with a wavelength of 434 nm ν = c/λ λ = 434 nm c = 3. 00 × 108 m/s This is the answer you should get: Solve for v ν = 6. 91 × 1014 Hz

Quantum Theory • • Planck (1900) • Observed - emission of light from hot objects • Concluded - energy is emitted in small, specific amounts (quanta) Quantum - minimum amount of energy change

Quantum Theory • Einstein (1905) • Observed - photoelectric effect • Concluded - light has properties of both waves and particles • • “wave-particle duality” Photon - particle of light that carries a quantum of energy

Photoelectric Effect • • photoelectric effect- emission of electrons from a metal when light shines on the metal. • no electrons were emitted if the light’s frequency was below a certain minimum regardless of lights intensity. • light form of energy able to knock loose an electron from a metal. wave theory of light- light any frequency could supply enough energy to eject an electron.

Quantum Theory • The energy of a photon is proportional to its frequency. E=h ν • E: energy (J, joules) • h: Planck’s constant(6. 6262 × 10 -34 J·s) • ν: frequency (Hz)

EX: Find the energy of a red photon with a frequency of 4. 57 × 1014 Hz. GIVEN: __________ E=? ν = 4. 57 × 1014 Hz h = 6. 6262 × 10 -34 J·s WORK: ___________ E = hν E = (6. 6262 × 10 -34 J·s) (4. 57 × 1014 Hz) E = 3. 03 × 10 -19 J

Bohr Model of an Atom

Bohr Model • e- exist only in orbits with specific amounts of energy called energy levels • Therefore… • e- can only gain or lose certain amounts of energy • only certain photons are produced

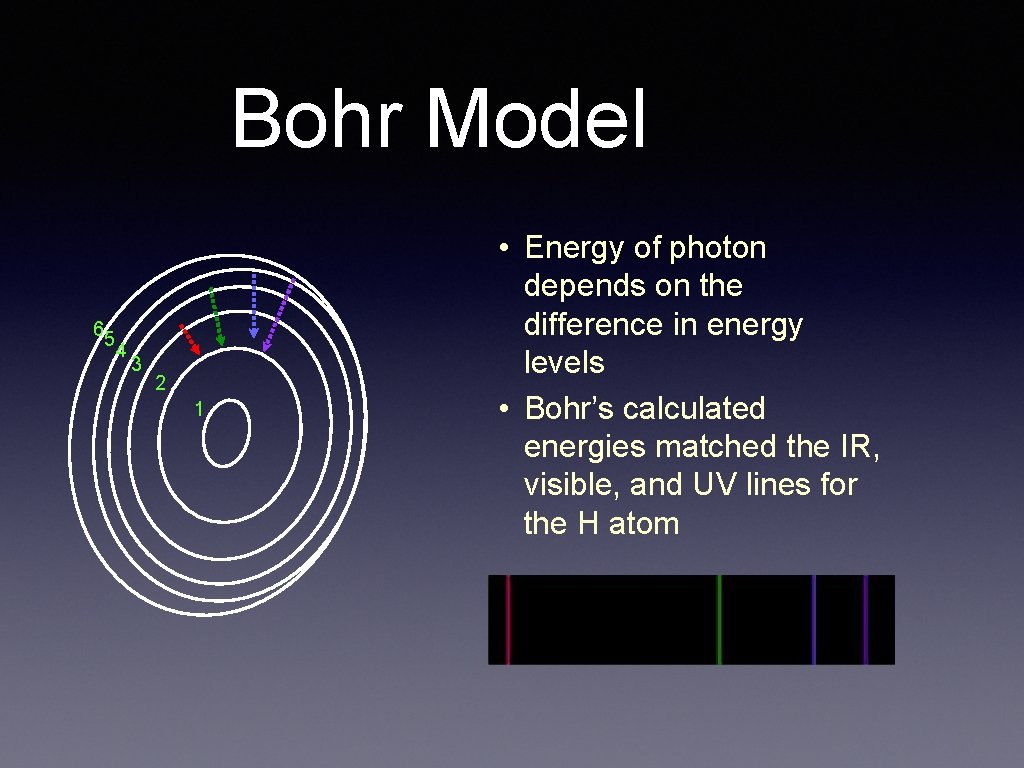
Bohr Model 65 4 3 2 1 • Energy of photon depends on the difference in energy levels • Bohr’s calculated energies matched the IR, visible, and UV lines for the H atom

Other Elements • Each element has a unique bright-line emission spectrum. • “Atomic Fingerprint” • Helium • Bohr’s calculations only worked for hydrogen! ☹