Waves Particles Electrons in Atoms Waves Wavelength length








- Slides: 20

Waves & Particles Electrons in Atoms

Waves • Wavelength ( ) - length of one complete wave • Frequency ( ) - # of waves that pass a point during a certain time period – hertz (Hz) = 1/s • Amplitude (A) - distance from the origin to the trough or crest

Waves crest A greater amplitude (intensity) origin A trough greater frequency (color)

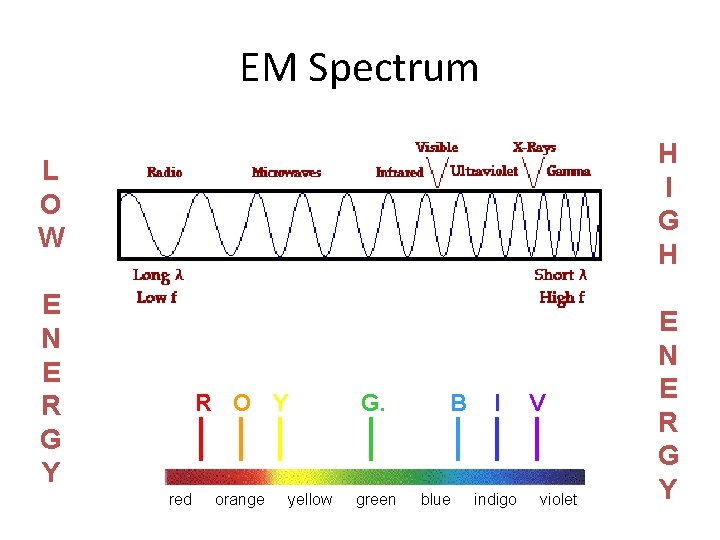
EM Spectrum H I G H L O W E N E R G Y red R O Y G. orange green yellow B blue I indigo V violet E N E R G Y


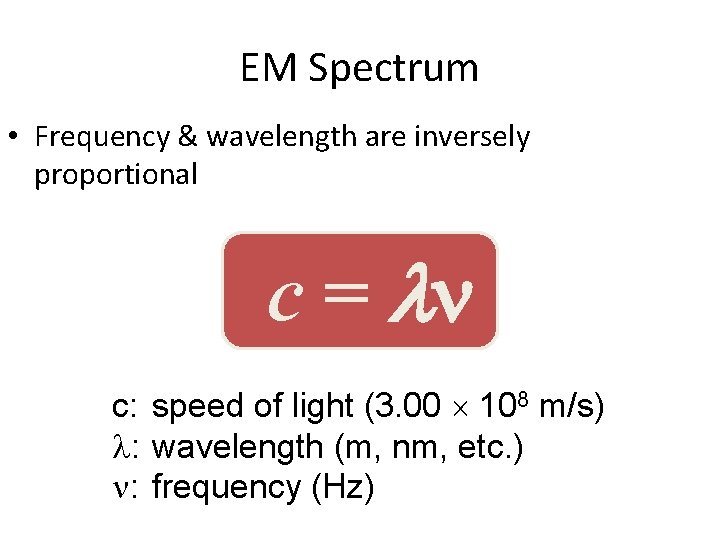
EM Spectrum • Frequency & wavelength are inversely proportional c = c: speed of light (3. 00 108 m/s) : wavelength (m, nm, etc. ) : frequency (Hz)

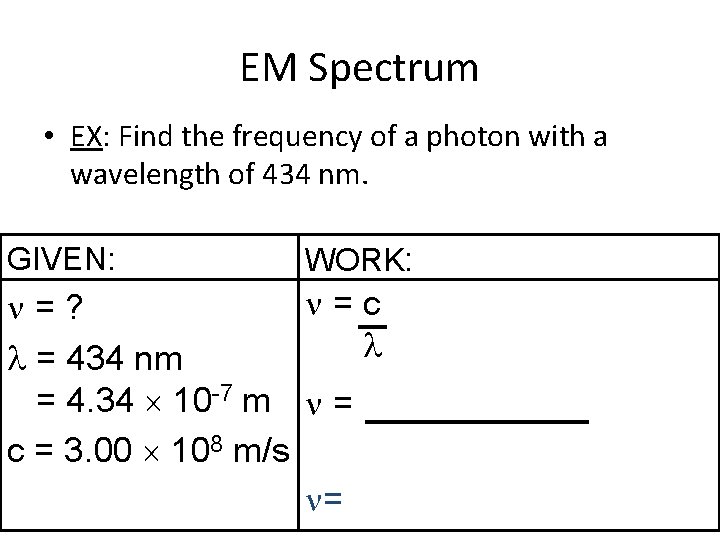
EM Spectrum • EX: Find the frequency of a photon with a wavelength of 434 nm. GIVEN: WORK: =c =? = 434 nm = 4. 34 10 -7 m = c = 3. 00 108 m/s =

Quantum Theory • Planck – Observed - emission of light from hot objects gave a rainbow but light from the emission of atoms was not. – Concluded - energy is emitted in small, specific amounts (quanta) – Quantum - minimum amount of energy change

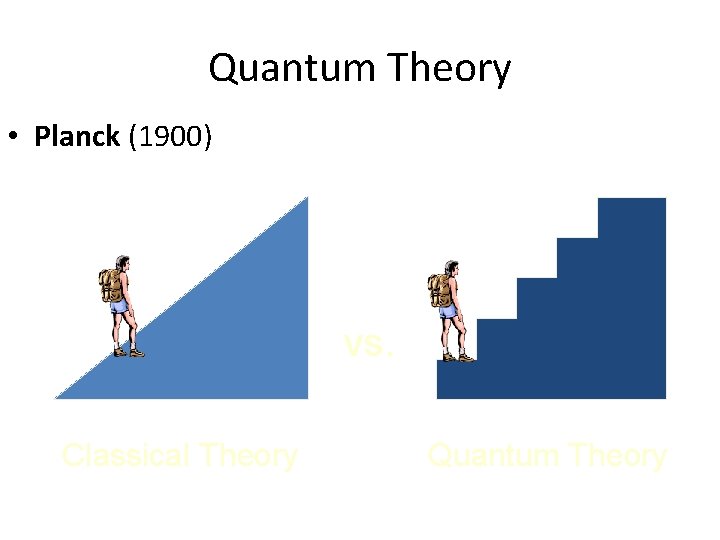
Quantum Theory • Planck (1900) vs. Classical Theory Quantum Theory

Line-Emission Spectrum excited state ENERGY IN PHOTON OUT ground state


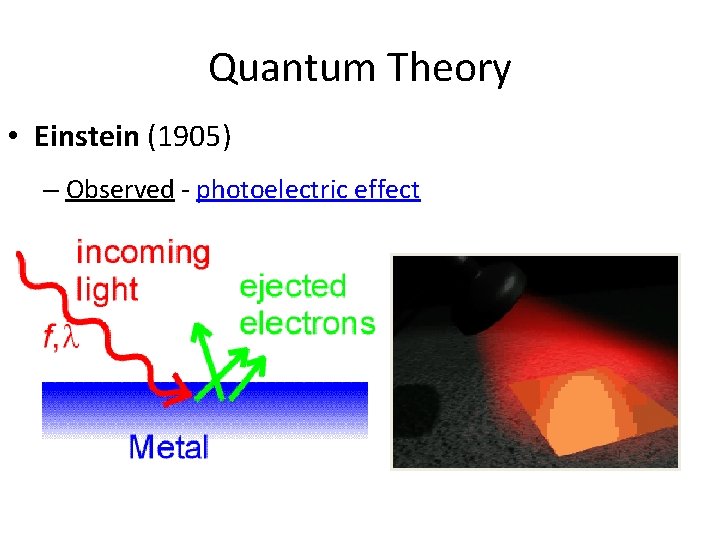
Quantum Theory • Einstein (1905) – Observed - photoelectric effect

Quantum Theory • Einstein (1905) – Concluded - light has properties of both waves and particles “wave-particle duality” – Photon - particle of light that carries a quantum of energy

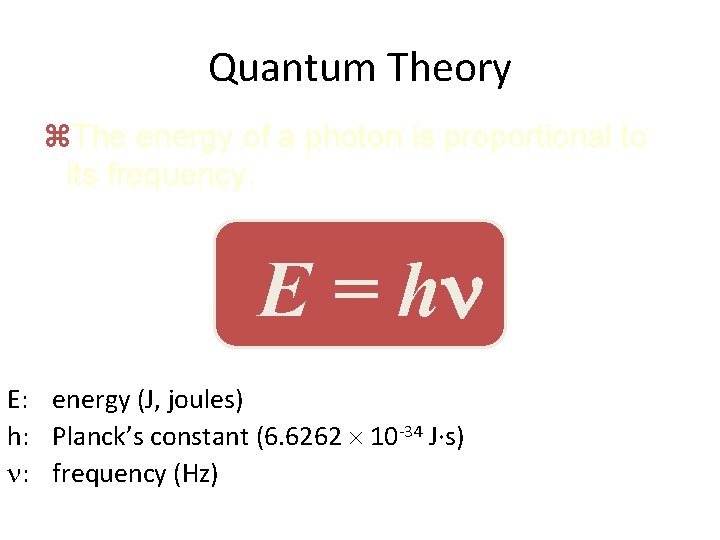
Quantum Theory z. The energy of a photon is proportional to its frequency. E = h E: energy (J, joules) h: Planck’s constant (6. 6262 10 -34 J·s) : frequency (Hz)

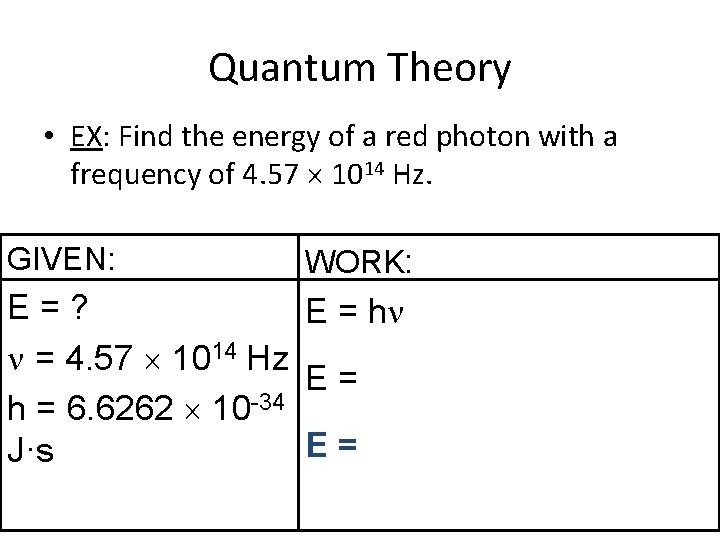
Quantum Theory • EX: Find the energy of a red photon with a frequency of 4. 57 1014 Hz. GIVEN: WORK: E=? E = h = 4. 57 1014 Hz E= h = 6. 6262 10 -34 E= J·s

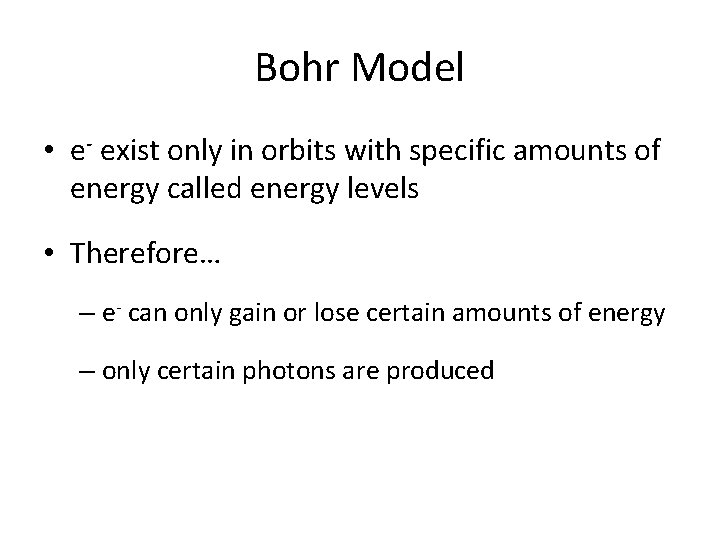
Bohr Model • e- exist only in orbits with specific amounts of energy called energy levels • Therefore… – e- can only gain or lose certain amounts of energy – only certain photons are produced

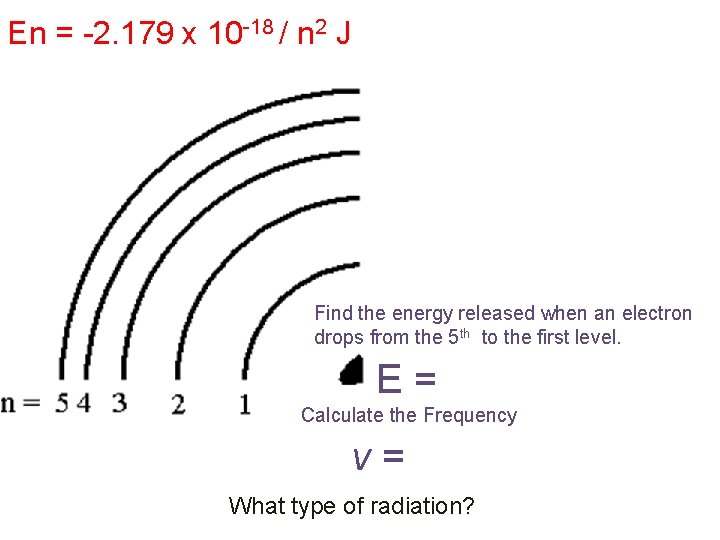
En = -2. 179 x 10 -18 / n 2 J Find the energy released when an electron drops from the 5 th to the first level. E= Calculate the Frequency v= What type of radiation?

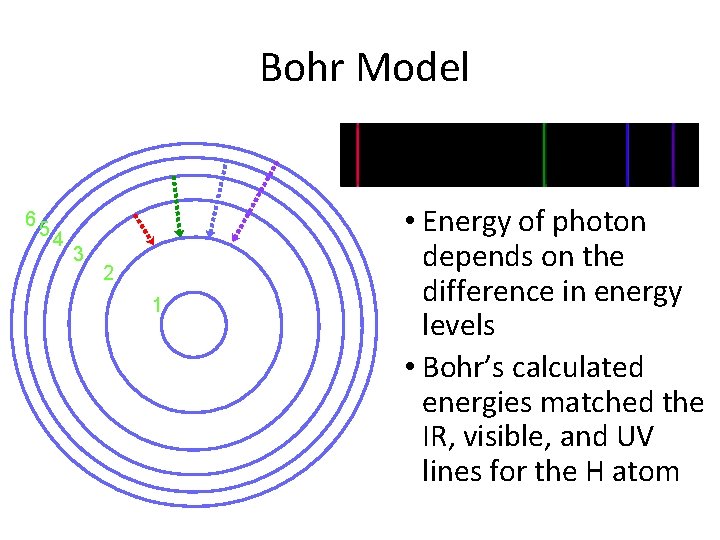
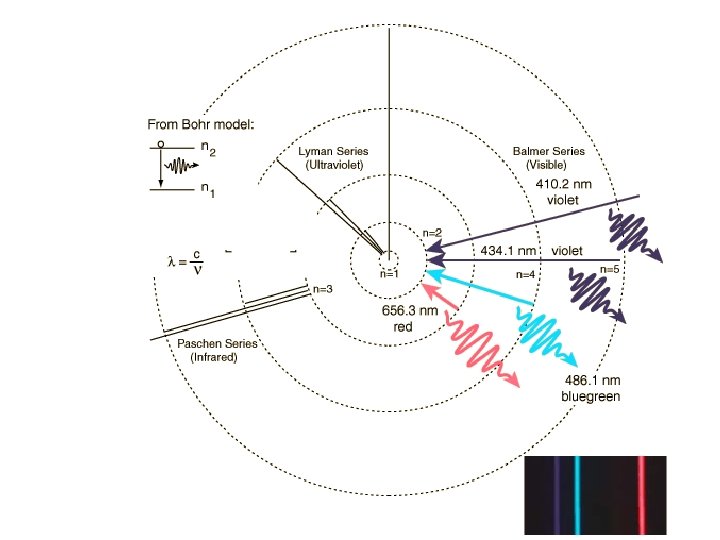
Bohr Model 65 4 3 2 1 • Energy of photon depends on the difference in energy levels • Bohr’s calculated energies matched the IR, visible, and UV lines for the H atom


Other Elements • Each element has a unique bright-line emission spectrum. – “Atomic Fingerprint” Helium z. Bohr’s calculations only worked for hydrogen!