Wedge dash to Newman Projection Newman projection is

- Slides: 32

Wedge- dash to Newman Projection Newman projection is instrumental in predicting the most stable conformer but molecular representation is done by wedge-dash method. Thus the conversion is essential to represent the most stable conformer. Sub-domain : Stereochemistry Sukumar Honkote Department of Chemistry, IIT Bombay

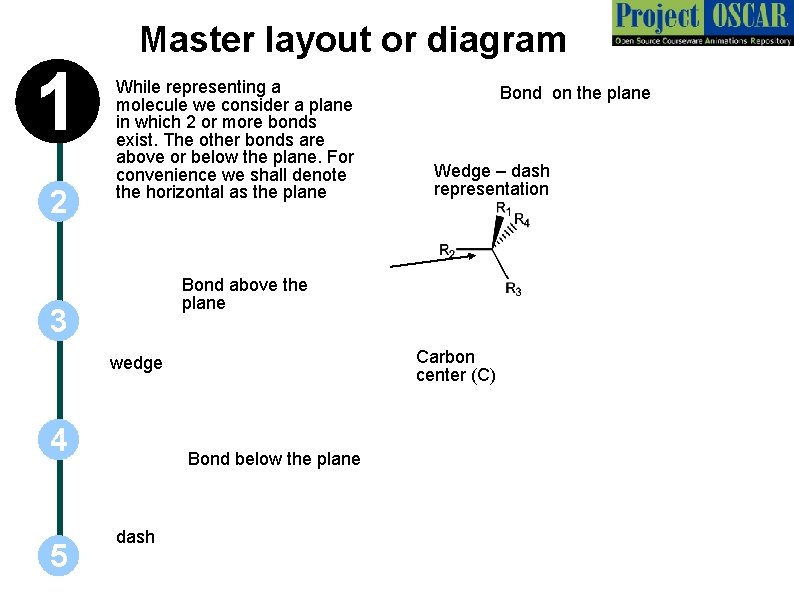
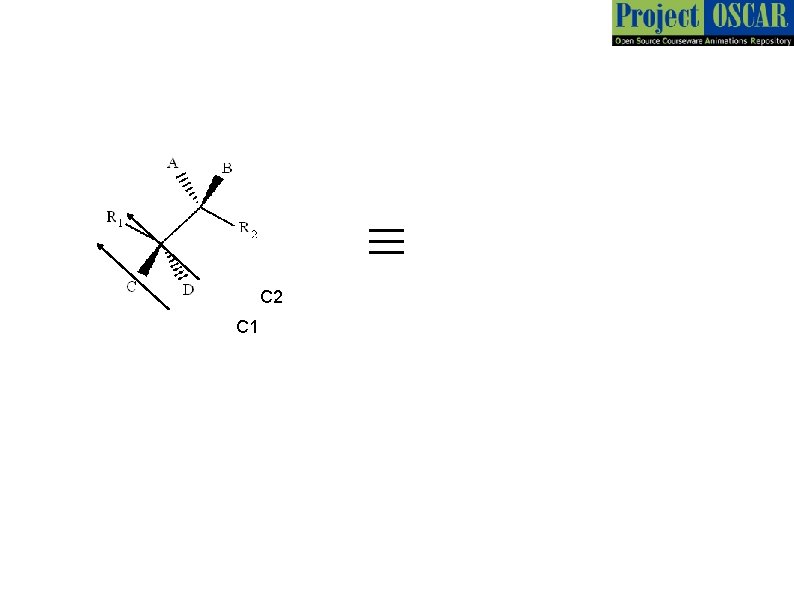
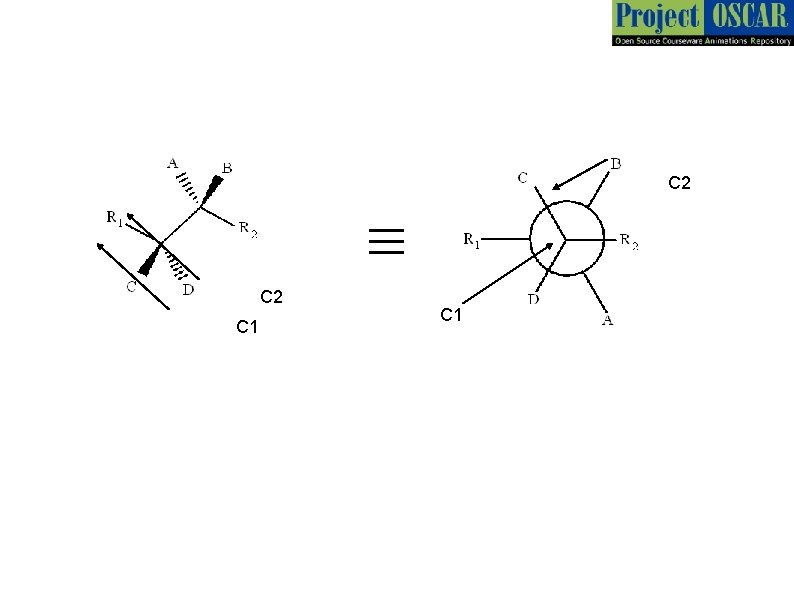
1 2 Master layout or diagram While representing a molecule we consider a plane in which 2 or more bonds exist. The other bonds are above or below the plane. For convenience we shall denote the horizontal as the plane Wedge – dash representation Bond above the plane 3 Carbon center (C) wedge 4 5 Bond on the plane Bond below the plane dash

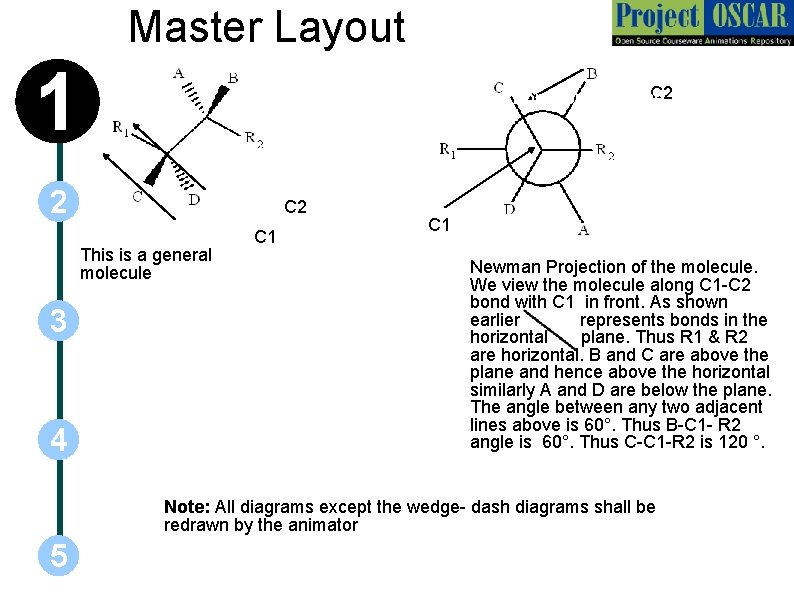
Master Layout 1 C 2 Bond on the plane 2 C 2 This is a general molecule 3 4 C 1 Newman Projection of the molecule. We view the molecule along C 1 -C 2 bond with C 1 in front. As shown earlier represents bonds in the horizontal plane. Thus R 1 & R 2 are horizontal. B and C are above the plane and hence above the horizontal similarly A and D are below the plane. The angle between any two adjacent lines above is 60°. Thus B-C 1 - R 2 angle is 60°. Thus C-C 1 -R 2 is 120 °. Note: All diagrams except the wedge- dash diagrams shall be redrawn by the animator 5

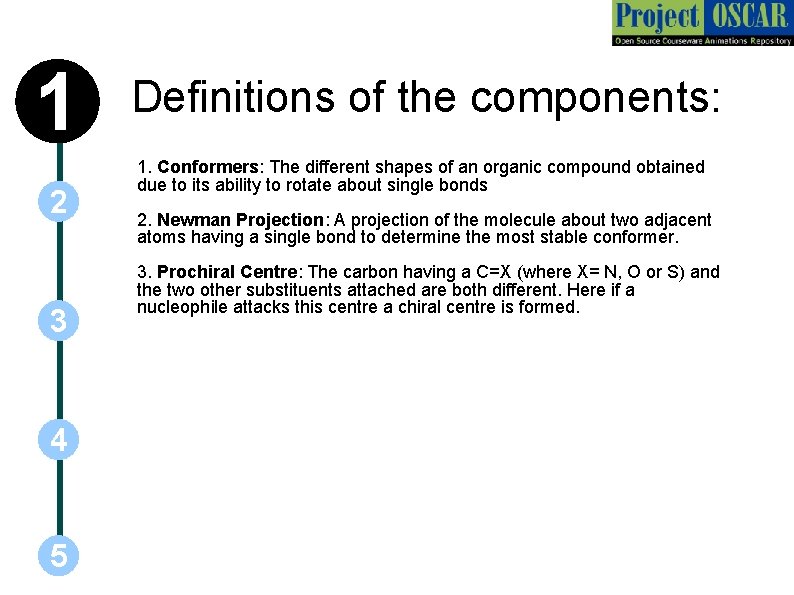
1 2 3 4 5 Definitions of the components: 1. Conformers: The different shapes of an organic compound obtained due to its ability to rotate about single bonds 2. Newman Projection: A projection of the molecule about two adjacent atoms having a single bond to determine the most stable conformer. 3. Prochiral Centre: The carbon having a C=X (where X= N, O or S) and the two other substituents attached are both different. Here if a nucleophile attacks this centre a chiral centre is formed.

1 2 Explain the process 1. The need of the concept shall be explained. 2. The scientific authenticity behind the concept is explained. 3. The animation will consist of 2 parts: a) with no double bond b) with a double bond. 4. The user will be given the choice initially. 5. After the user selects one choice he is shown how the conversion is done correctly. 6. Then he is given the choice again to select one of the two parts. He can replay the previous part or select the other part. 7. There will be option for the questionnaire. 3 4 Click shift + F 5 from the next slide to view how the animation should be Note: It should be mentioned that the prerequisite for this animation is conformational analysis. 5

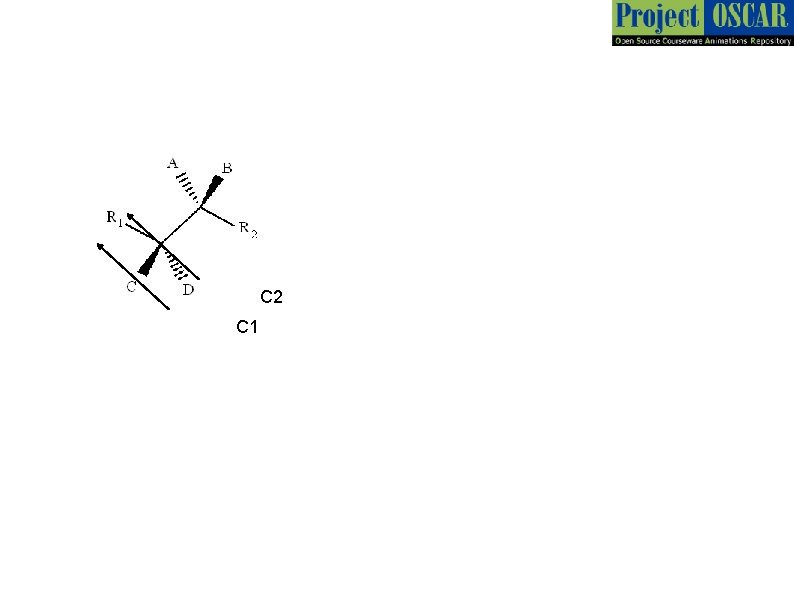
C 2 C 1

≡ C 2 C 1

C 2 ≡ C 2 C 1

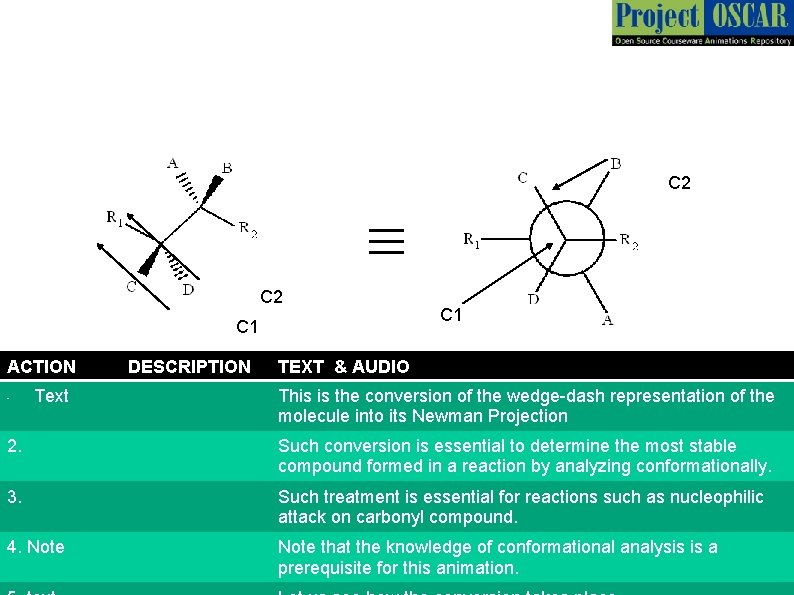
C 2 ≡ C 2 C 1 ACTION • Text DESCRIPTION C 1 TEXT & AUDIO This is the conversion of the wedge-dash representation of the molecule into its Newman Projection 2. Such conversion is essential to determine the most stable compound formed in a reaction by analyzing conformationally. 3. Such treatment is essential for reactions such as nucleophilic attack on carbonyl compound. 4. Note that the knowledge of conformational analysis is a prerequisite for this animation.

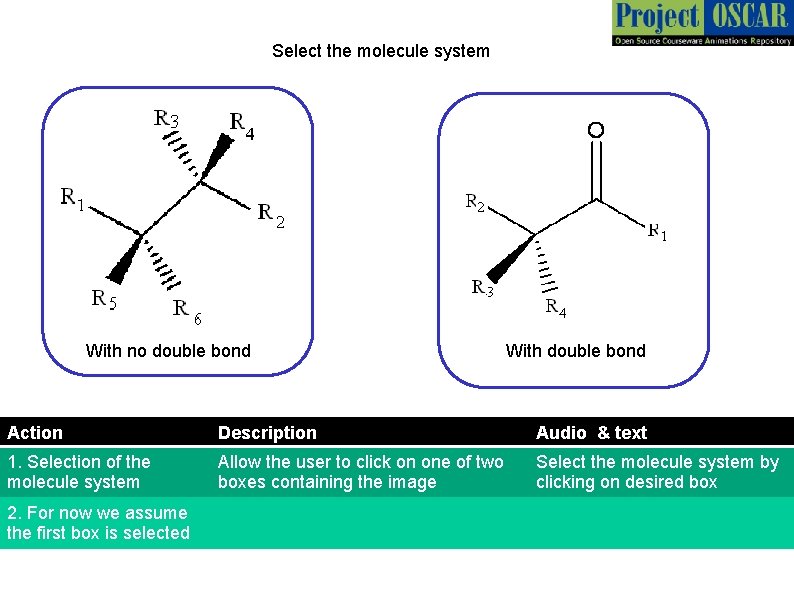
Select the molecule system With no double bond With double bond Action Description Audio & text 1. Selection of the molecule system Allow the user to click on one of two boxes containing the image Select the molecule system by clicking on desired box 2. For now we assume the first box is selected

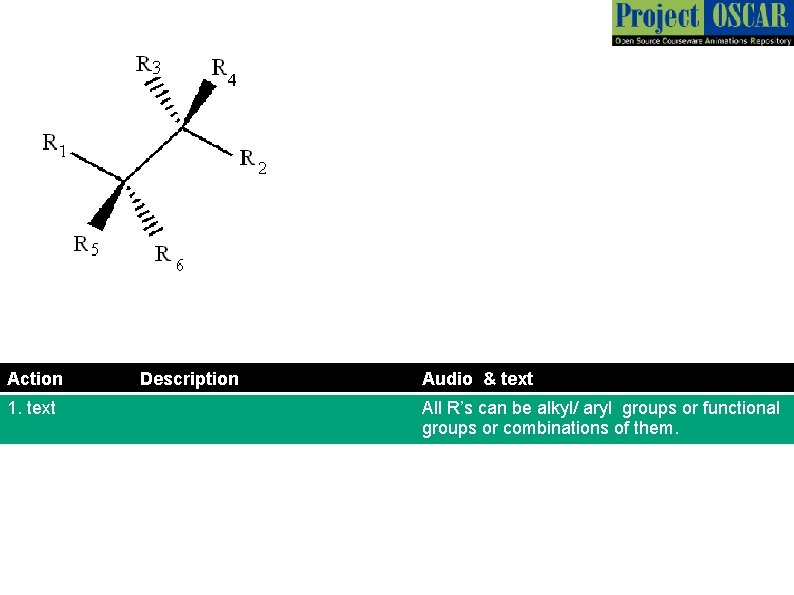
Action 1. text Description Audio & text All R’s can be alkyl/ aryl groups or functional groups or combinations of them.

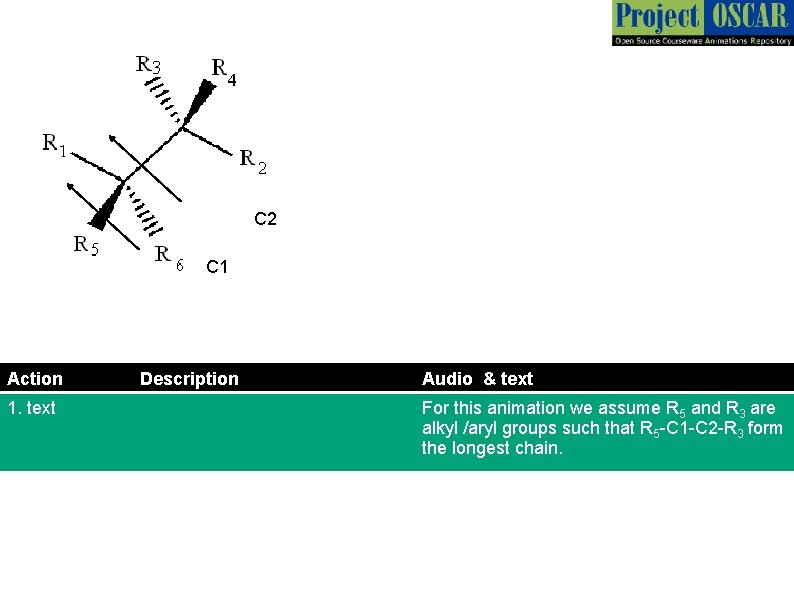
C 2 C 1 Action 1. text Description Audio & text For this animation we assume R 5 and R 3 are alkyl /aryl groups such that R 5 -C 1 -C 2 -R 3 form the longest chain.

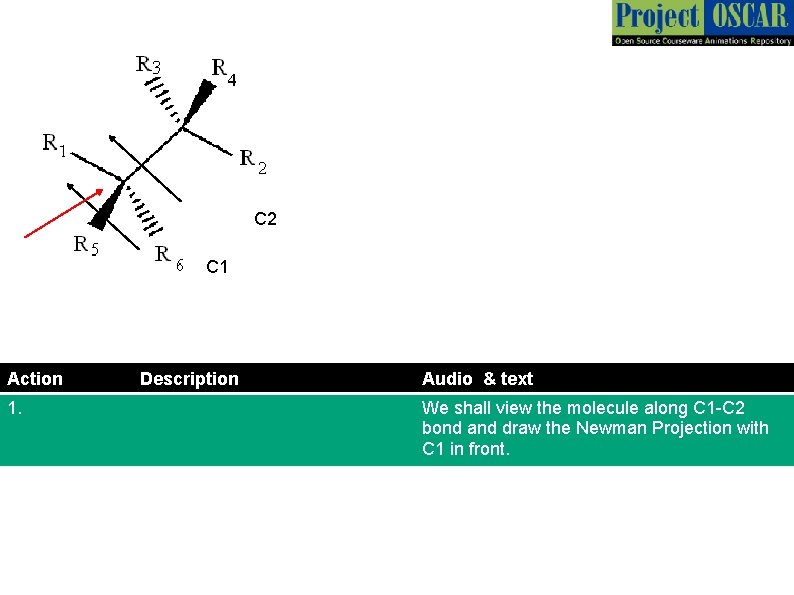
C 2 C 1 Action 1. Description Audio & text We shall view the molecule along C 1 -C 2 bond and draw the Newman Projection with C 1 in front.

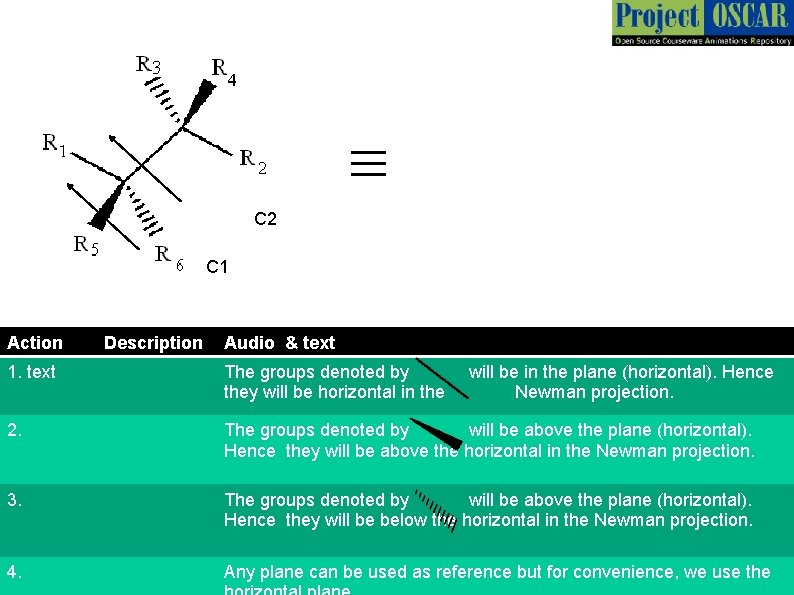
≡ C 2 C 1 Action Description Audio & text 1. text The groups denoted by they will be horizontal in the will be in the plane (horizontal). Hence Newman projection. 2. The groups denoted by will be above the plane (horizontal). Hence they will be above the horizontal in the Newman projection. 3. The groups denoted by will be above the plane (horizontal). Hence they will be below the horizontal in the Newman projection. 4. Any plane can be used as reference but for convenience, we use the

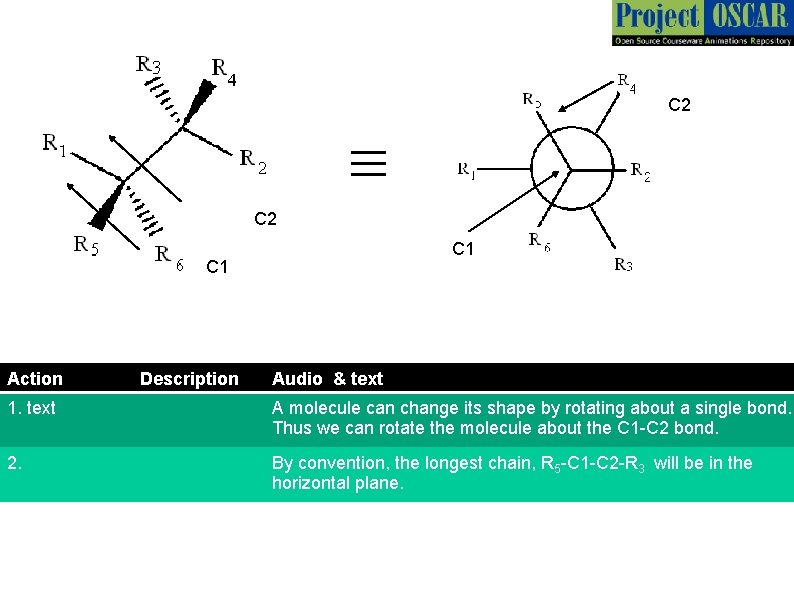
C 2 ≡ C 2 C 1 Action Description Audio & text 1. text A molecule can change its shape by rotating about a single bond. Thus we can rotate the molecule about the C 1 -C 2 bond. 2. By convention, the longest chain, R 5 -C 1 -C 2 -R 3 will be in the horizontal plane.

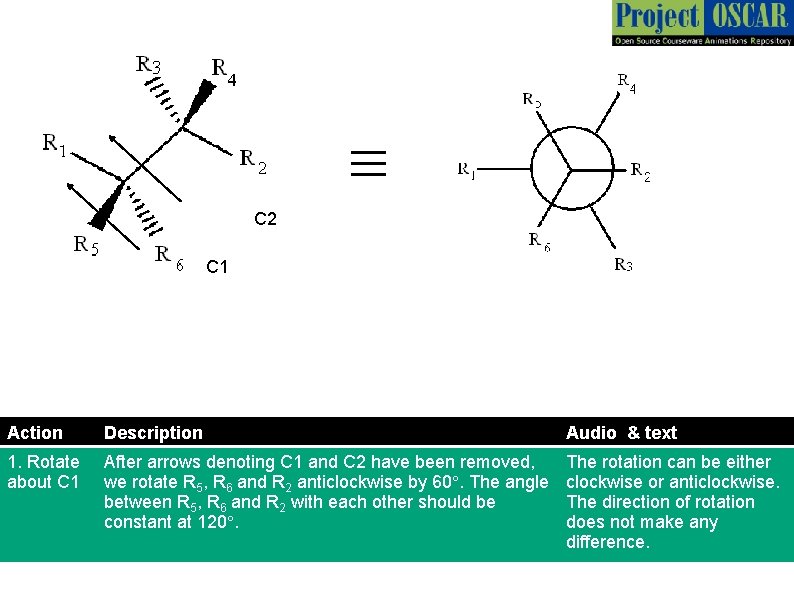
≡ C 2 C 1 Action Description Audio & text 1. Rotate about C 1 After arrows denoting C 1 and C 2 have been removed, we rotate R 5, R 6 and R 2 anticlockwise by 60°. The angle between R 5, R 6 and R 2 with each other should be constant at 120°. The rotation can be either clockwise or anticlockwise. The direction of rotation does not make any difference.

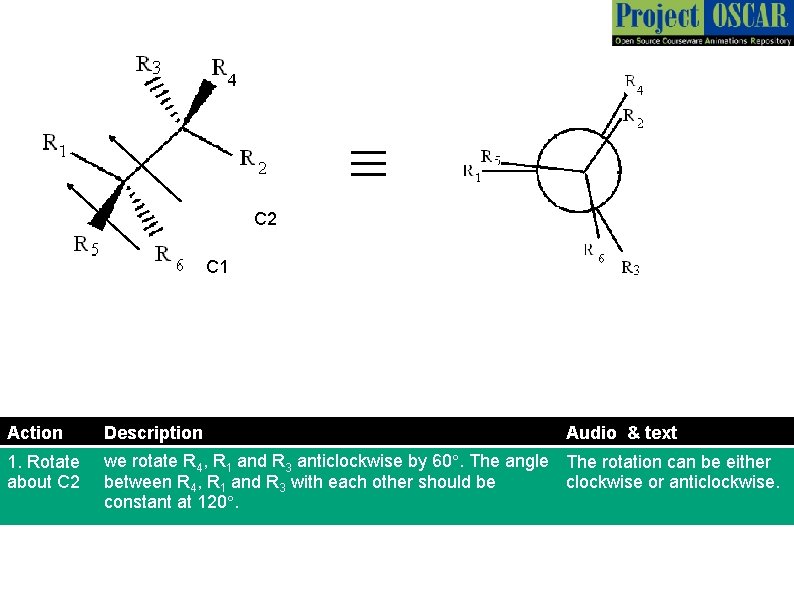
≡ C 2 C 1 Action Description Audio & text 1. Rotate about C 2 we rotate R 4, R 1 and R 3 anticlockwise by 60°. The angle The rotation can be either between R 4, R 1 and R 3 with each other should be clockwise or anticlockwise. constant at 120°.

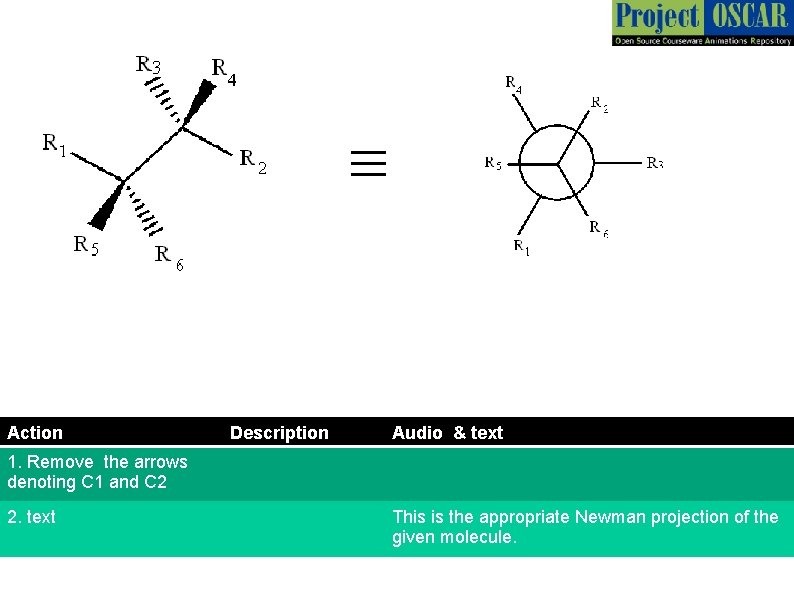
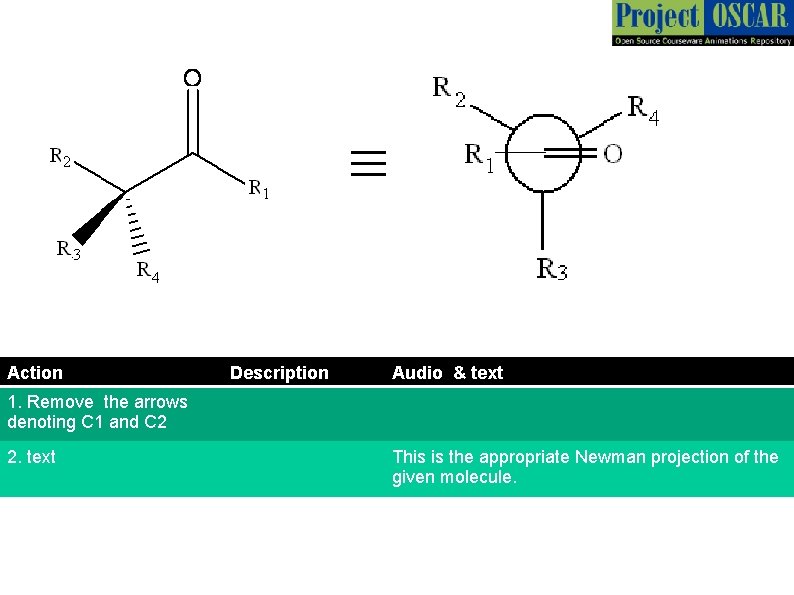
≡ Action Description Audio & text 1. Remove the arrows denoting C 1 and C 2 2. text This is the appropriate Newman projection of the given molecule.

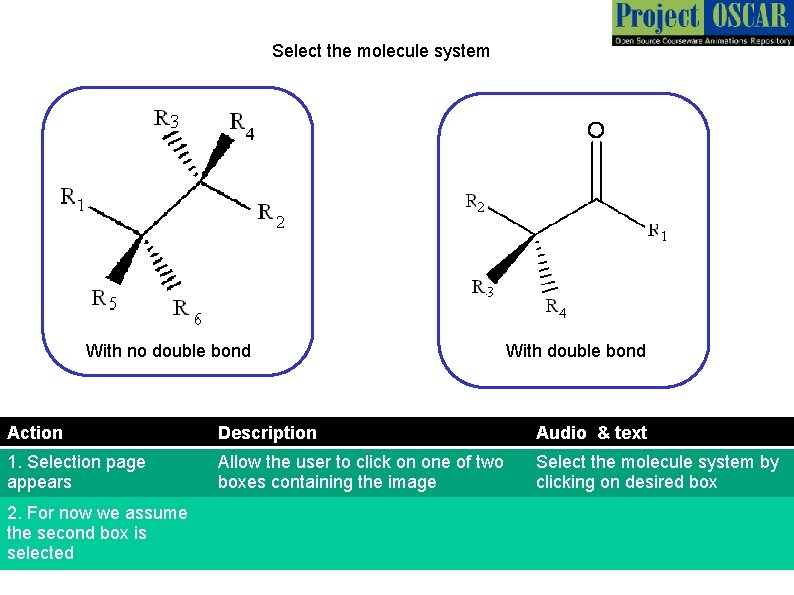
Select the molecule system With no double bond With double bond Action Description Audio & text 1. Selection page appears Allow the user to click on one of two boxes containing the image Select the molecule system by clicking on desired box 2. For now we assume the second box is selected

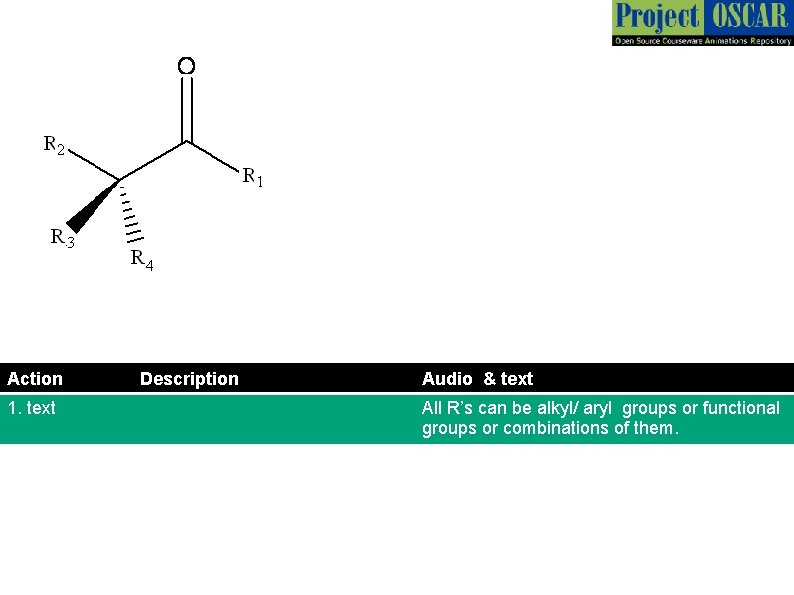
Action 1. text Description Audio & text All R’s can be alkyl/ aryl groups or functional groups or combinations of them.

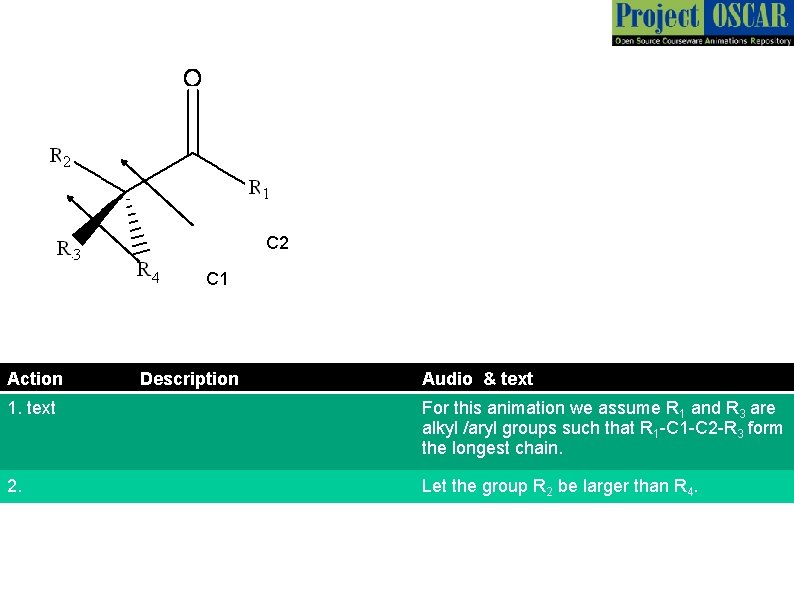
C 2 C 1 Action Description Audio & text 1. text For this animation we assume R 1 and R 3 are alkyl /aryl groups such that R 1 -C 2 -R 3 form the longest chain. 2. Let the group R 2 be larger than R 4.

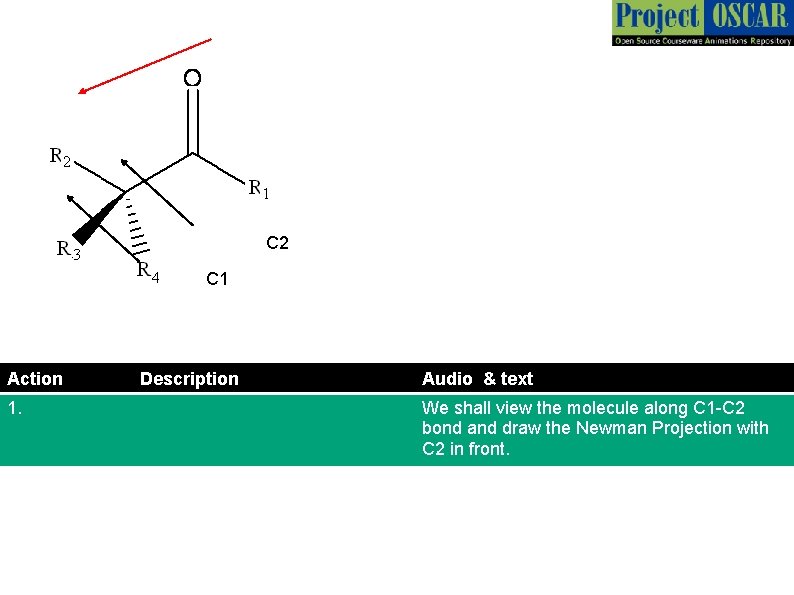
C 2 C 1 Action 1. Description Audio & text We shall view the molecule along C 1 -C 2 bond and draw the Newman Projection with C 2 in front.

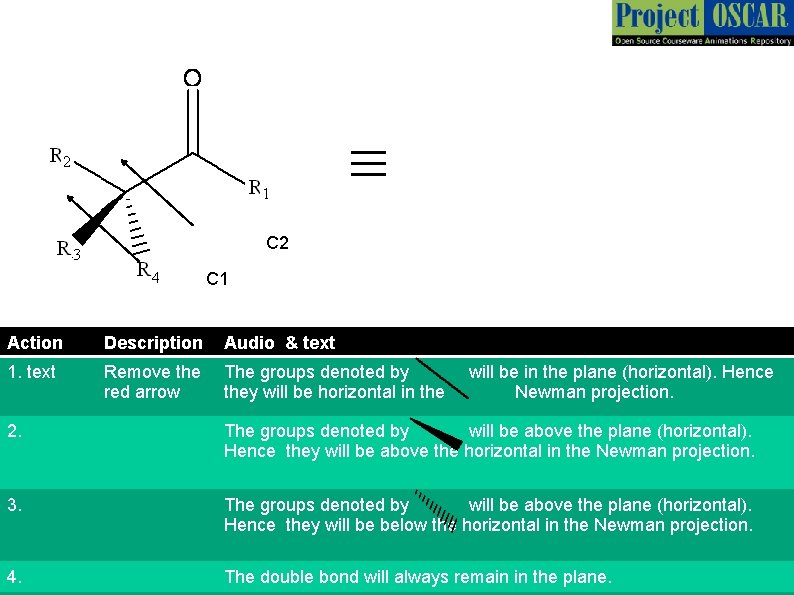
≡ C 2 C 1 Action Description 1. text Action Remove the The groups denoted by Audiowill in the plane (horizontal). Hence Description & be text red arrow they will be horizontal in the Newman projection. For this animation we assume R 1 and R 3 are groups such R 1(horizontal). -C 1 -C 2 -R 3 form The groups denoted by alkyl /aryl will be above that plane chain. Hence they will be abovethe thelongest horizontal in the Newman projection. 1. text 2. Audio & text 2. 3. We shall view the molecule along C 1 -C 2 The groups denoted by bond and will be above the plane. Projection (horizontal). draw the Newman with Hence they will be below. C 1 theinhorizontal in the Newman projection. front. 4. The double bond will always remain in the plane.

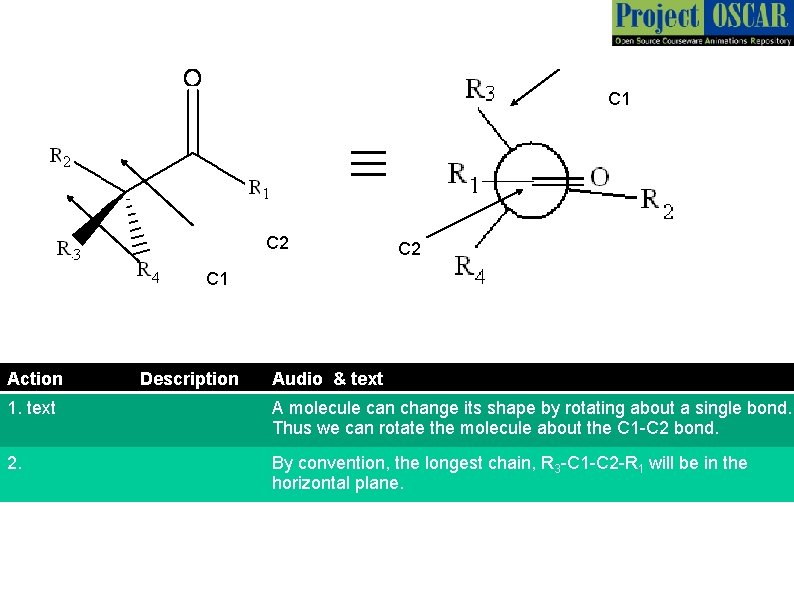
C 1 ≡ C 2 C 1 Action Description C 2 Audio & text 1. text A molecule can change its shape by rotating about a single bond. Thus we can rotate the molecule about the C 1 -C 2 bond. 2. By convention, the longest chain, R 3 -C 1 -C 2 -R 1 will be in the horizontal plane.

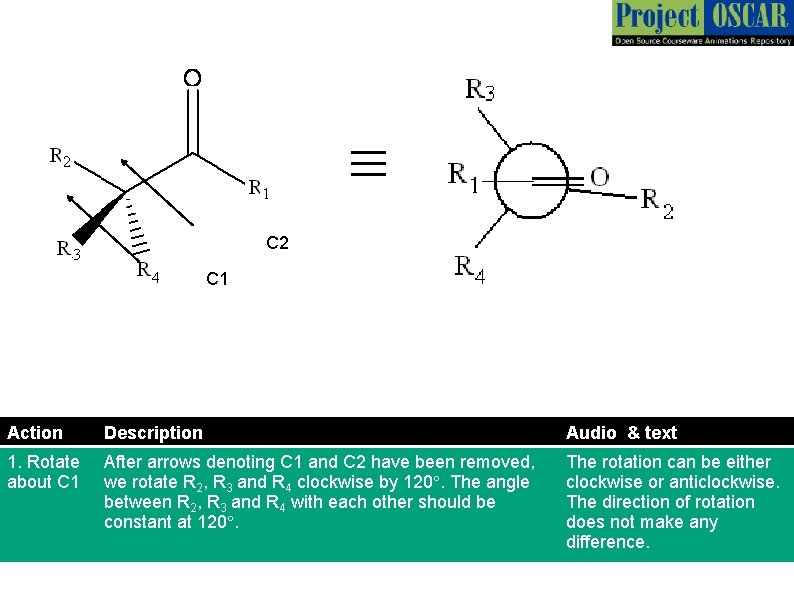
≡ C 2 C 1 Action Description Audio & text 1. Rotate about C 1 After arrows denoting C 1 and C 2 have been removed, we rotate R 2, R 3 and R 4 clockwise by 120°. The angle between R 2, R 3 and R 4 with each other should be constant at 120°. The rotation can be either clockwise or anticlockwise. The direction of rotation does not make any difference.

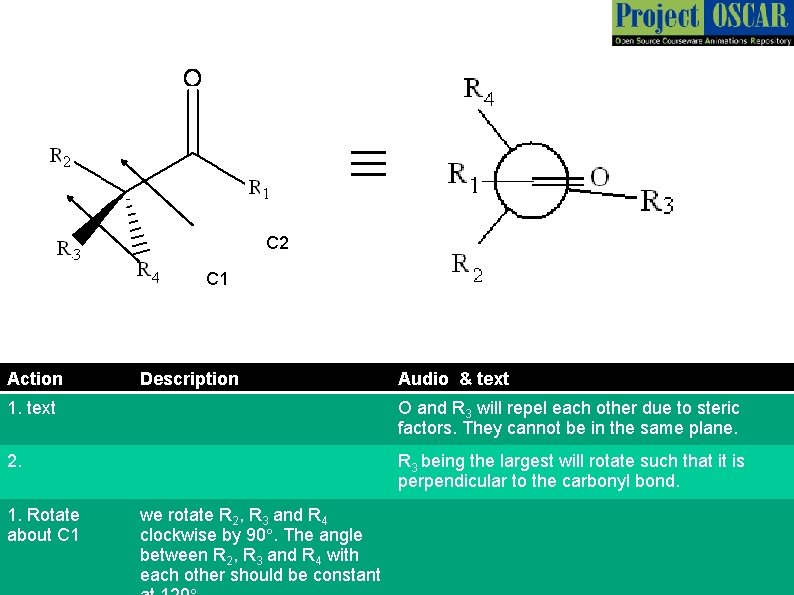
≡ C 2 C 1 Action Description Audio & text 1. text O and R 3 will repel each other due to steric factors. They cannot be in the same plane. 2. R 3 being the largest will rotate such that it is perpendicular to the carbonyl bond. 1. Rotate about C 1 we rotate R 2, R 3 and R 4 clockwise by 90°. The angle between R 2, R 3 and R 4 with each other should be constant

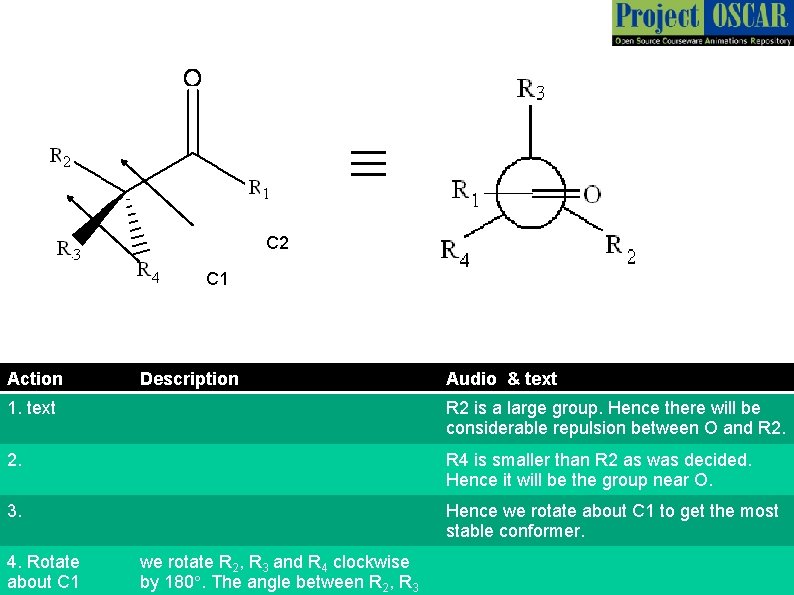
≡ C 2 C 1 Action Description Audio & text 1. text R 2 is a large group. Hence there will be considerable repulsion between O and R 2. R 4 is smaller than R 2 as was decided. Hence it will be the group near O. 3. Hence we rotate about C 1 to get the most stable conformer. 4. Rotate about C 1 we rotate R 2, R 3 and R 4 clockwise by 180°. The angle between R 2, R 3

≡ C 2 C 1 Action Description Audio & text 1. Remove the arrows denoting C 1 and C 2 2. text This is the appropriate Newman projection of the given molecule.

References books 1) Organic Chemistry Jonathan Clayden, Nick Greeves, Stuart Warren, and Peter Wothers 2) Solomons, Fryhle: Organic Chemistry, 8 th Edition

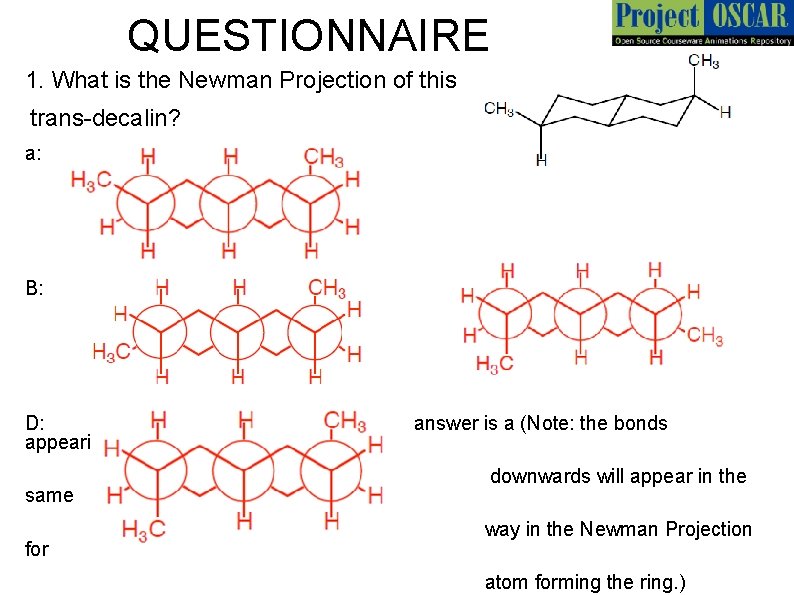
QUESTIONNAIRE 1. What is the Newman Projection of this trans-decalin? a: B: D: appearing axially upwards or same for c: answer is a (Note: the bonds downwards will appear in the way in the Newman Projection atom forming the ring. )

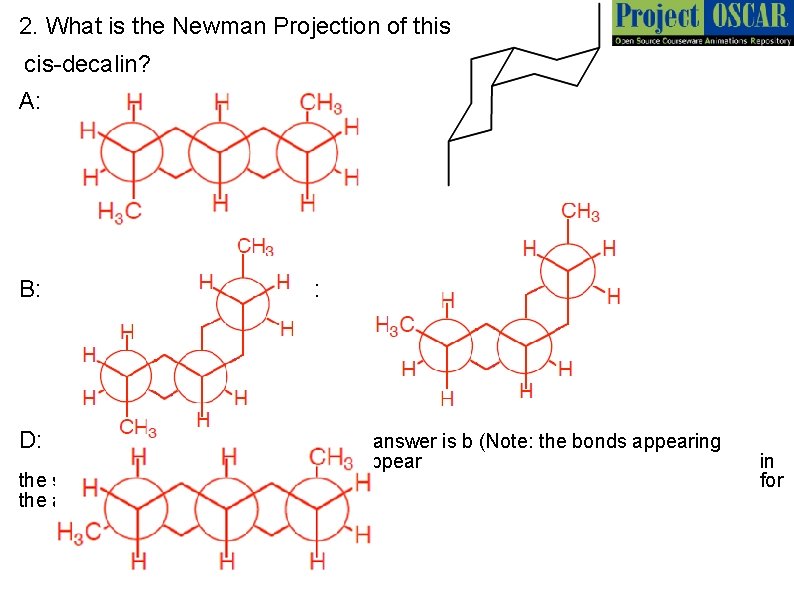
2. What is the Newman Projection of this cis-decalin? A: B: D: c: answer is b (Note: the bonds appearing axially upwards or downwards will appear the same way in the Newman Projection the atoms forming the ring) in for

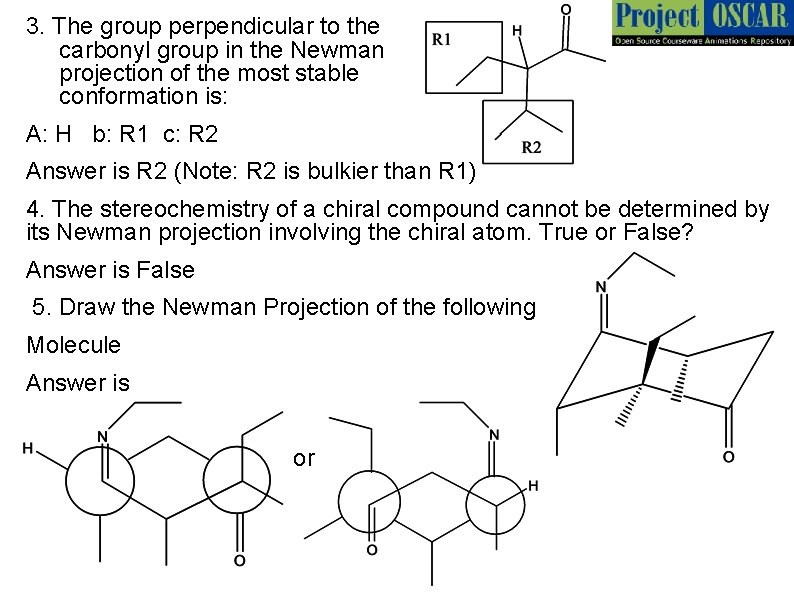
3. The group perpendicular to the carbonyl group in the Newman projection of the most stable conformation is: A: H b: R 1 c: R 2 Answer is R 2 (Note: R 2 is bulkier than R 1) 4. The stereochemistry of a chiral compound cannot be determined by its Newman projection involving the chiral atom. True or False? Answer is False 5. Draw the Newman Projection of the following Molecule Answer is or
 Dash vs wedge
Dash vs wedge How to convert wedge dash to fischer
How to convert wedge dash to fischer D-glyceraldehyde wedge and dash
D-glyceraldehyde wedge and dash En dash vs em dash
En dash vs em dash Comma vs dash
Comma vs dash Newman projections practice
Newman projections practice Newman projection generator
Newman projection generator C3h6 newman projection
C3h6 newman projection Z and e configuration
Z and e configuration Isometric drawing from orthographic view
Isometric drawing from orthographic view Third angle
Third angle Third angle system
Third angle system Receding axis
Receding axis Scalar vs vector projection
Scalar vs vector projection Ryan lang art
Ryan lang art Alcanos formulas
Alcanos formulas Barnett newman vir heroicus sublimis
Barnett newman vir heroicus sublimis Gerald levey and mark newman
Gerald levey and mark newman Newman harvard referencing
Newman harvard referencing Girvan newman algorithm
Girvan newman algorithm Butano newman
Butano newman Geography hsc syllabus
Geography hsc syllabus Ciclo paul newman
Ciclo paul newman Minimal art 1960
Minimal art 1960 David newman carrot
David newman carrot Barnett newman abraham
Barnett newman abraham Milkovich newman compensation, 9th edition pdf
Milkovich newman compensation, 9th edition pdf Richard newman uf
Richard newman uf Teoria x y y de la administracion
Teoria x y y de la administracion Cognitive apprenticeship collins brown and newman
Cognitive apprenticeship collins brown and newman Gerald levey and mark newman
Gerald levey and mark newman Cardinal newman cedar
Cardinal newman cedar Gerald levey and mark newman
Gerald levey and mark newman