Stereochemistry Smita Asthana St Anns 1 1 FlyingWedge









- Slides: 38

Stereochemistry Smita Asthana @ St. Ann's 1

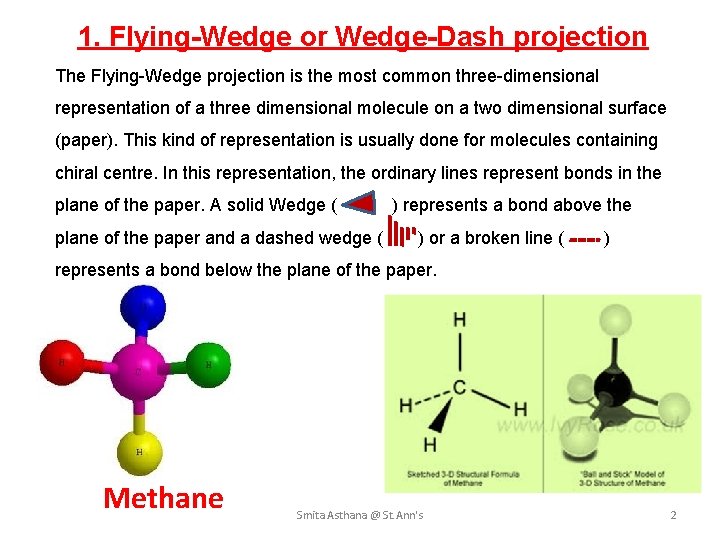
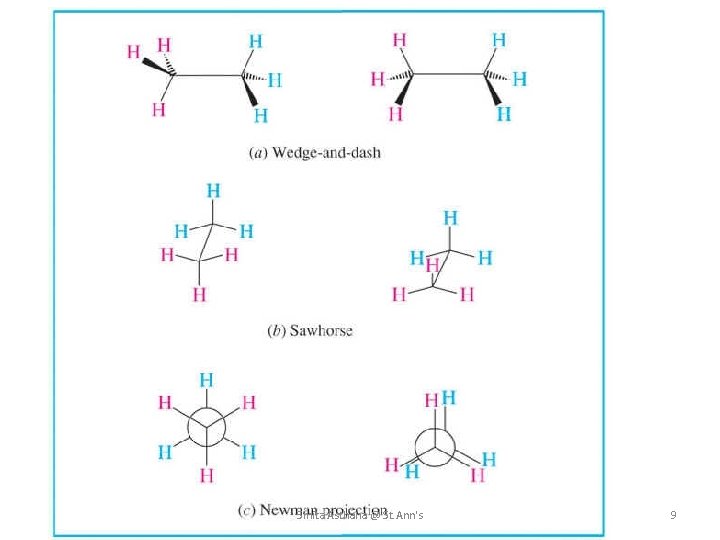
1. Flying-Wedge or Wedge-Dash projection The Flying-Wedge projection is the most common three-dimensional representation of a three dimensional molecule on a two dimensional surface (paper). This kind of representation is usually done for molecules containing chiral centre. In this representation, the ordinary lines represent bonds in the plane of the paper. A solid Wedge ( ) represents a bond above the plane of the paper and a dashed wedge ( ) or a broken line ( ) represents a bond below the plane of the paper. Methane Smita Asthana @ St. Ann's 2

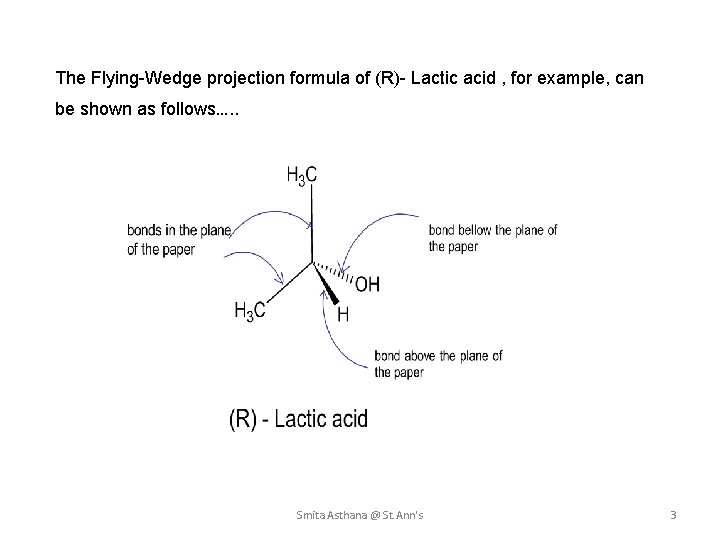
The Flying-Wedge projection formula of (R)- Lactic acid , for example, can be shown as follows…. . Smita Asthana @ St. Ann's 3

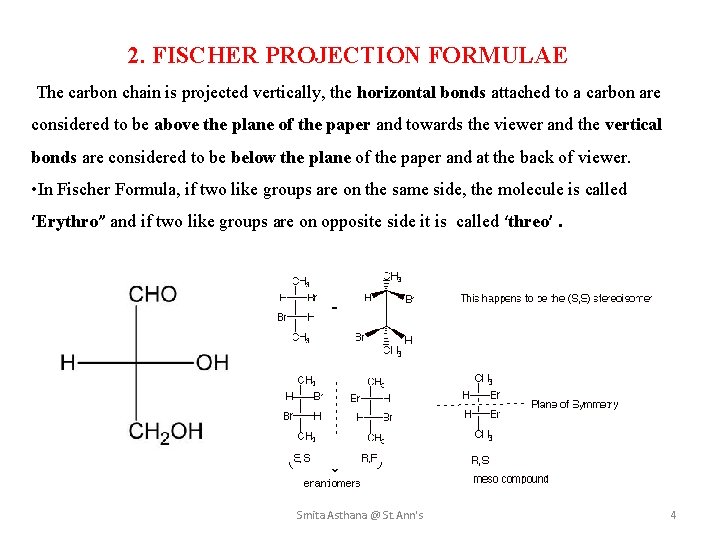
2. FISCHER PROJECTION FORMULAE The carbon chain is projected vertically, the horizontal bonds attached to a carbon are considered to be above the plane of the paper and towards the viewer and the vertical bonds are considered to be below the plane of the paper and at the back of viewer. • In Fischer Formula, if two like groups are on the same side, the molecule is called ‘Erythro” and if two like groups are on opposite side it is called ‘threo’. Smita Asthana @ St. Ann's 4

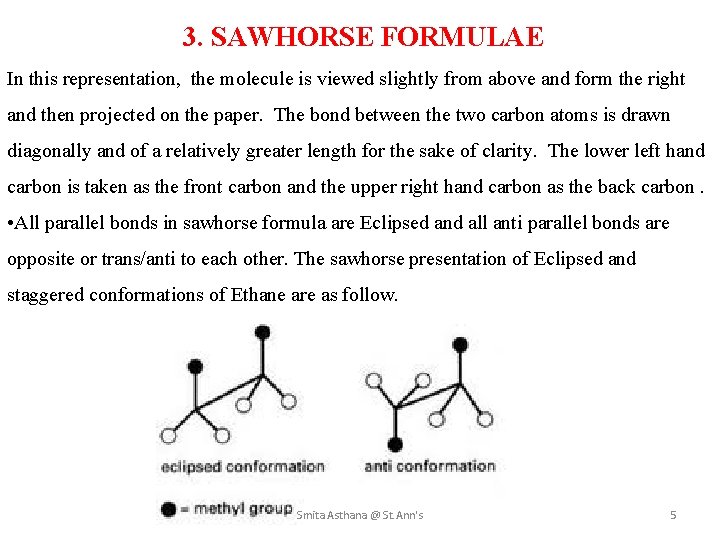
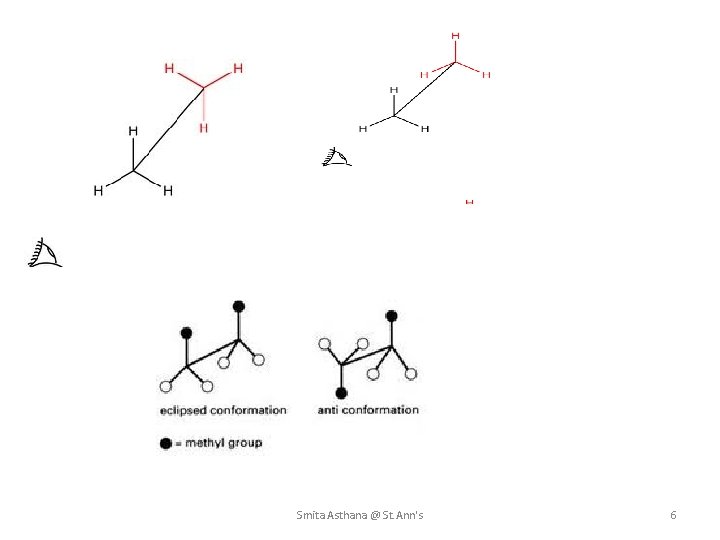
3. SAWHORSE FORMULAE In this representation, the molecule is viewed slightly from above and form the right and then projected on the paper. The bond between the two carbon atoms is drawn diagonally and of a relatively greater length for the sake of clarity. The lower left hand carbon is taken as the front carbon and the upper right hand carbon as the back carbon. • All parallel bonds in sawhorse formula are Eclipsed and all anti parallel bonds are opposite or trans/anti to each other. The sawhorse presentation of Eclipsed and staggered conformations of Ethane are as follow. Smita Asthana @ St. Ann's 5

Smita Asthana @ St. Ann's 6

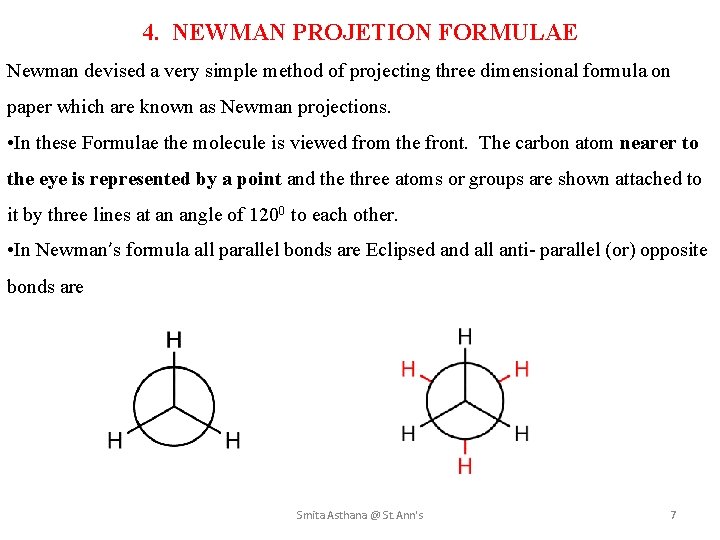
4. NEWMAN PROJETION FORMULAE Newman devised a very simple method of projecting three dimensional formula on paper which are known as Newman projections. • In these Formulae the molecule is viewed from the front. The carbon atom nearer to the eye is represented by a point and the three atoms or groups are shown attached to it by three lines at an angle of 1200 to each other. • In Newman’s formula all parallel bonds are Eclipsed and all anti- parallel (or) opposite bonds are Smita Asthana @ St. Ann's 7

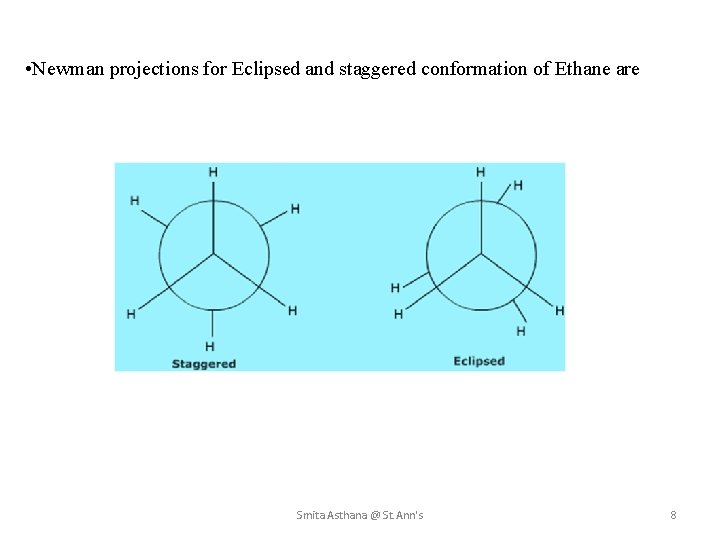
• Newman projections for Eclipsed and staggered conformation of Ethane are Smita Asthana @ St. Ann's 8

Smita Asthana @ St. Ann's 9

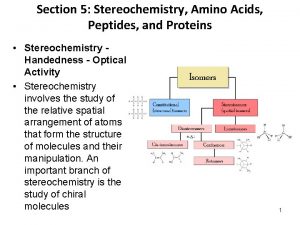
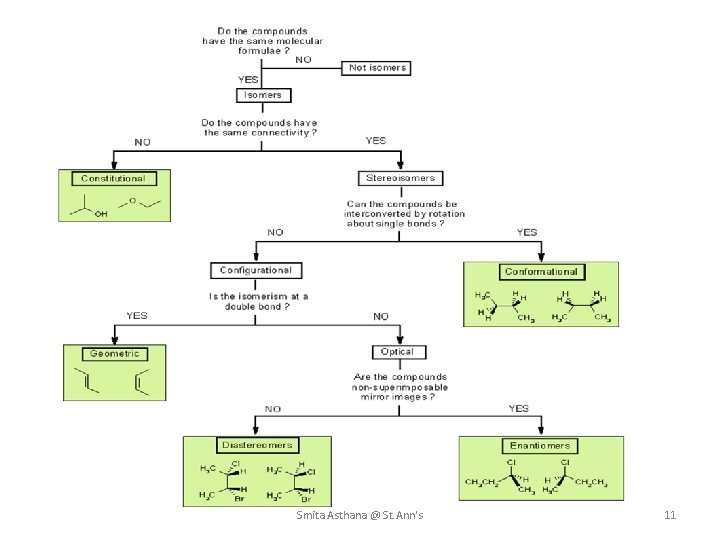
ISOMERISM Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. Stereoisomers. In stereoisomerism, the atoms making up the isomers are joined up in the same order, but still manage to have a different spatial arrangement. Smita Asthana @ St. Ann's 10

Smita Asthana @ St. Ann's 11

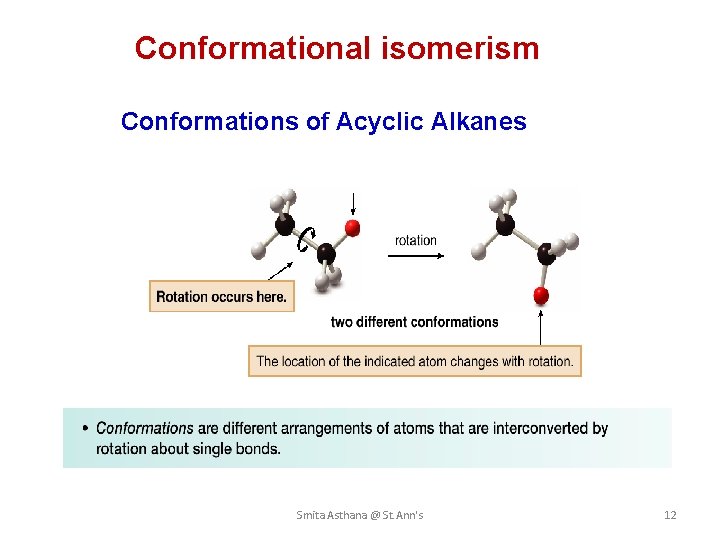
Conformational isomerism Conformations of Acyclic Alkanes Smita Asthana @ St. Ann's 12

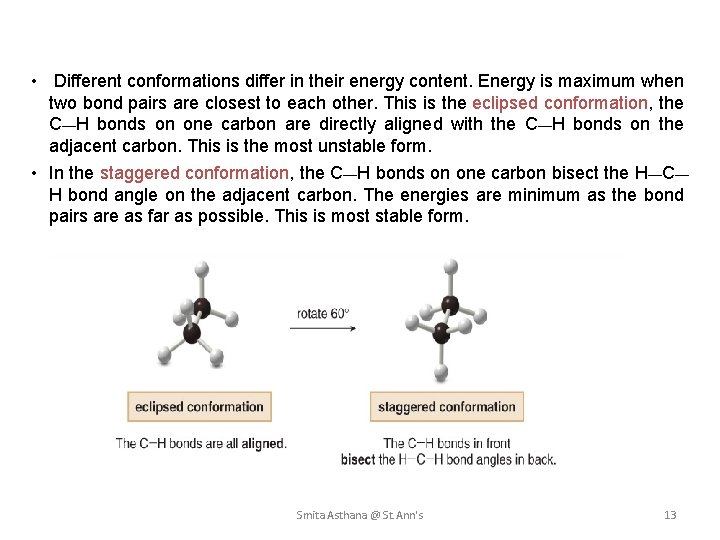
• Different conformations differ in their energy content. Energy is maximum when two bond pairs are closest to each other. This is the eclipsed conformation, the C—H bonds on one carbon are directly aligned with the C—H bonds on the adjacent carbon. This is the most unstable form. • In the staggered conformation, the C—H bonds on one carbon bisect the H—C— H bond angle on the adjacent carbon. The energies are minimum as the bond pairs are as far as possible. This is most stable form. Smita Asthana @ St. Ann's 13

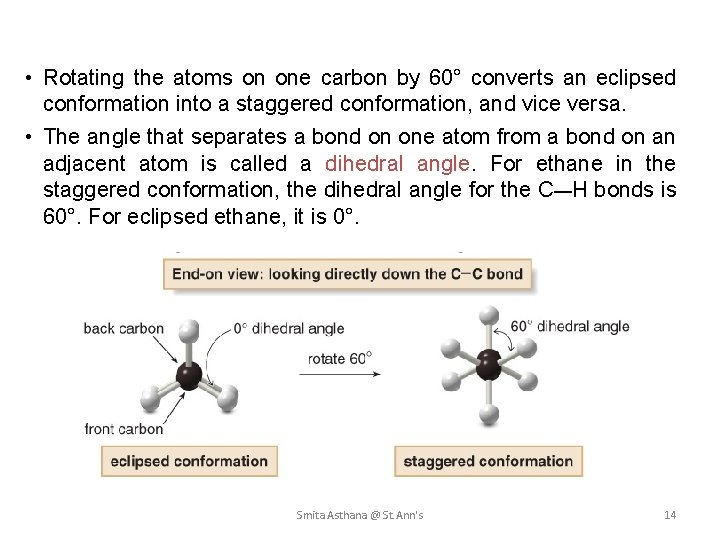
• Rotating the atoms on one carbon by 60° converts an eclipsed conformation into a staggered conformation, and vice versa. • The angle that separates a bond on one atom from a bond on an adjacent atom is called a dihedral angle. For ethane in the staggered conformation, the dihedral angle for the C—H bonds is 60°. For eclipsed ethane, it is 0°. Smita Asthana @ St. Ann's 14

Cnformational Analysis of Ethane • Conformations are different spatial arrangements of a molecule that are generated by rotation about single bonds. Smita Asthana @ St. Ann's 15

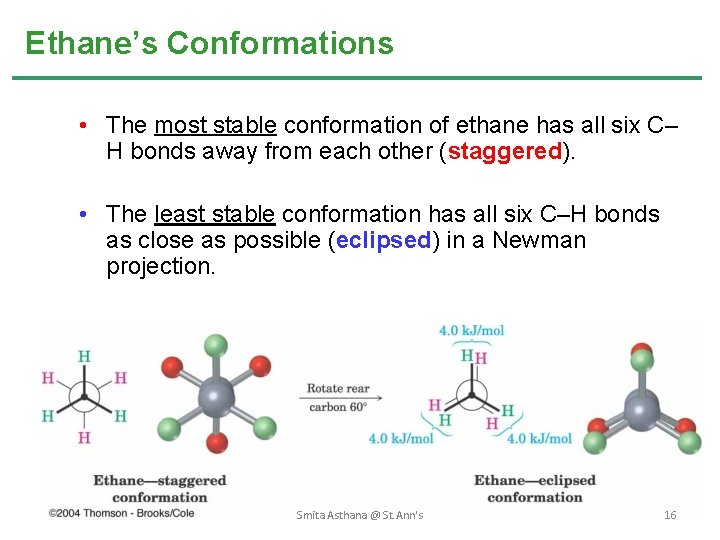
Ethane’s Conformations • The most stable conformation of ethane has all six C– H bonds away from each other (staggered). • The least stable conformation has all six C–H bonds as close as possible (eclipsed) in a Newman projection. Smita Asthana @ St. Ann's 16

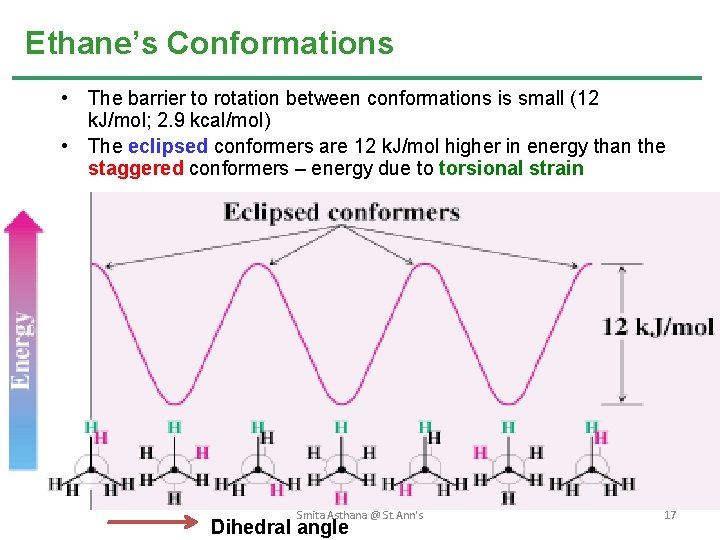
Ethane’s Conformations • The barrier to rotation between conformations is small (12 k. J/mol; 2. 9 kcal/mol) • The eclipsed conformers are 12 k. J/mol higher in energy than the staggered conformers – energy due to torsional strain Smita Asthana @ St. Ann's Dihedral angle 17

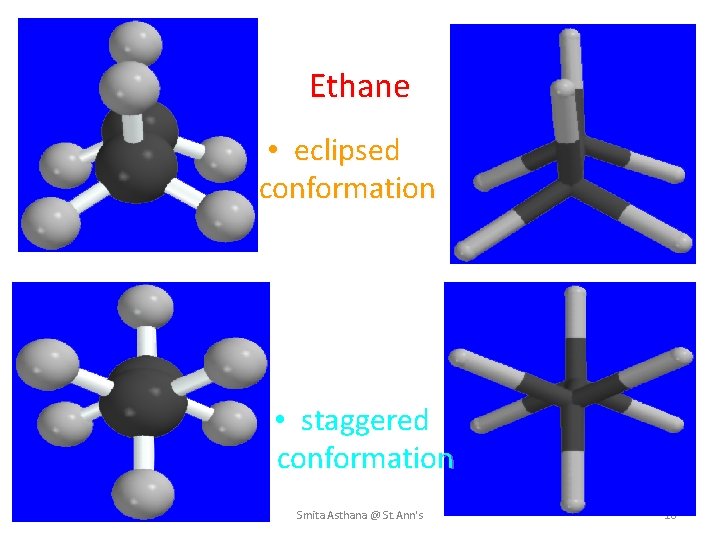
Ethane • eclipsed conformation • staggered conformation Smita Asthana @ St. Ann's 18

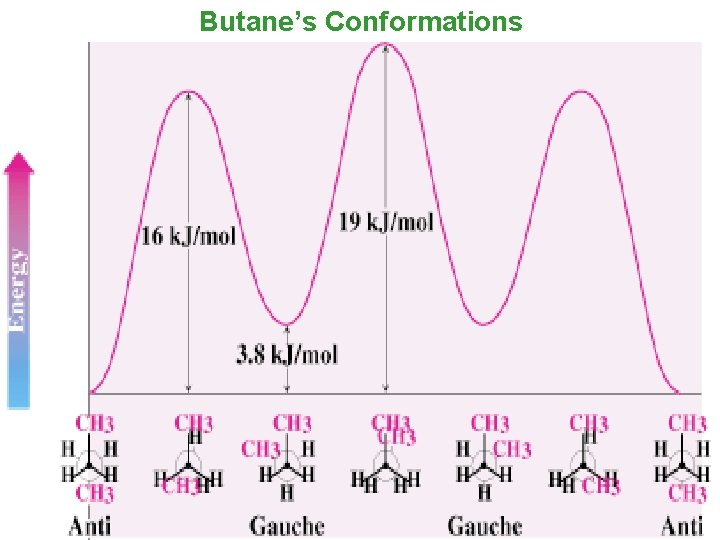
Conformational Analysis of Butane Smita Asthana @ St. Ann's 19

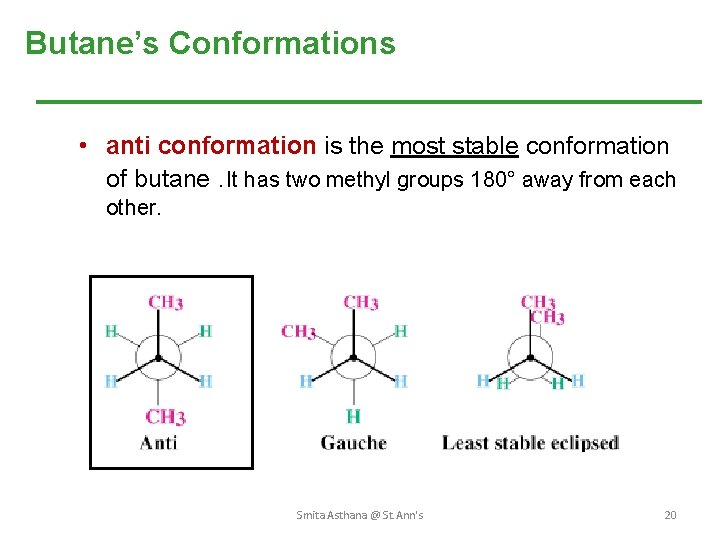
Butane’s Conformations • anti conformation is the most stable conformation of butane. It has two methyl groups 180° away from each other. Smita Asthana @ St. Ann's 20

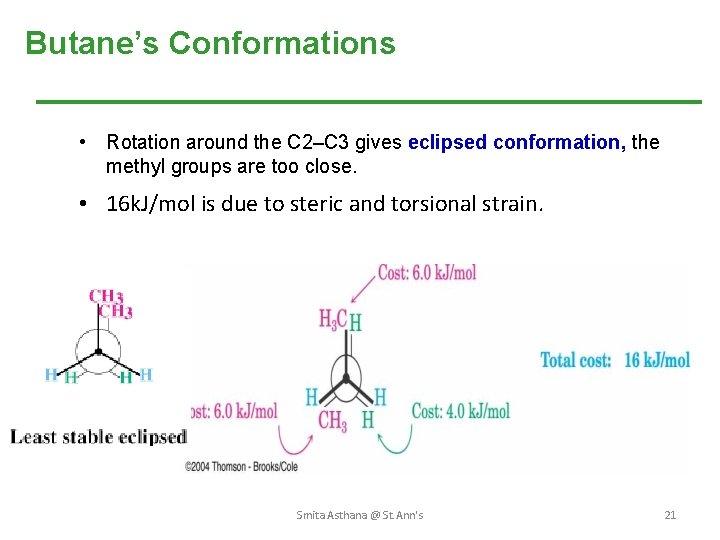
Butane’s Conformations • Rotation around the C 2–C 3 gives eclipsed conformation, the methyl groups are too close. • 16 k. J/mol is due to steric and torsional strain. Smita Asthana @ St. Ann's 21

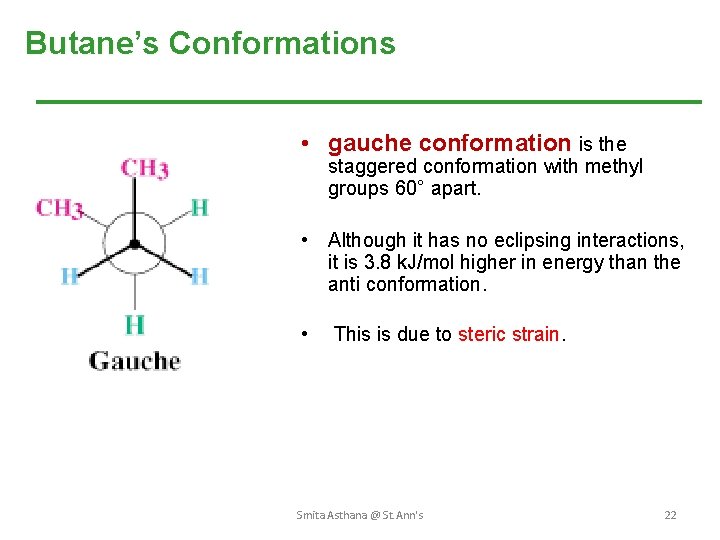
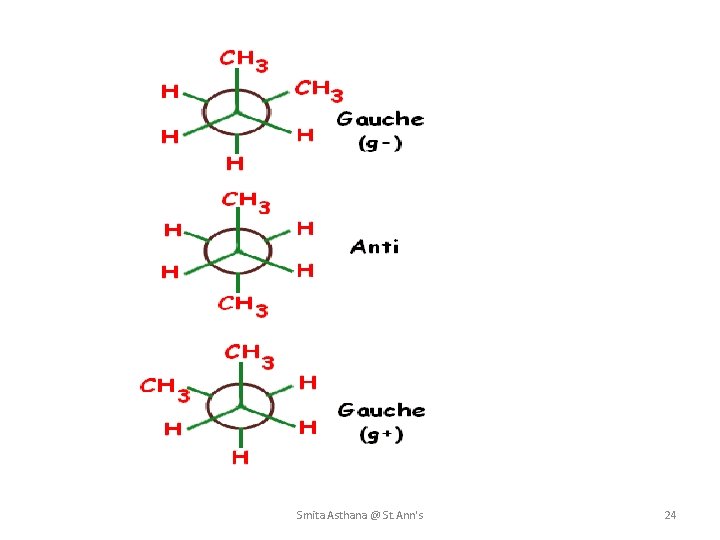
Butane’s Conformations • gauche conformation is the staggered conformation with methyl groups 60° apart. • Although it has no eclipsing interactions, it is 3. 8 k. J/mol higher in energy than the anti conformation. • This is due to steric strain. Smita Asthana @ St. Ann's 22

Butane’s Conformations Smita Asthana @ St. Ann's 23

Smita Asthana @ St. Ann's 24

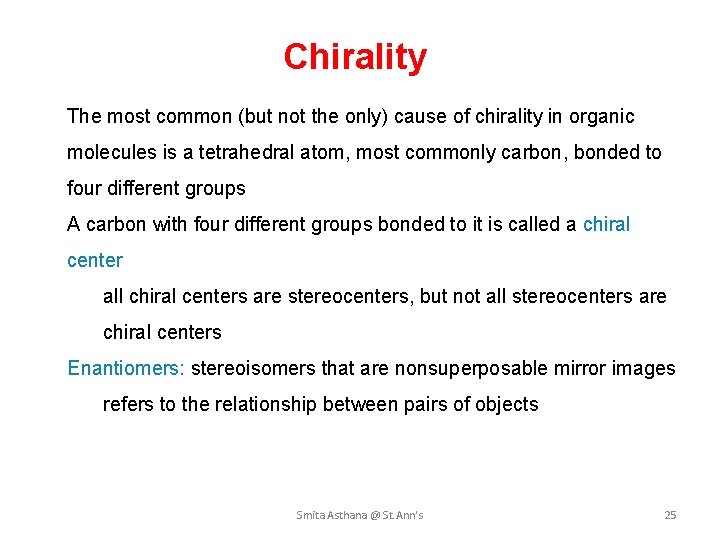
Chirality The most common (but not the only) cause of chirality in organic molecules is a tetrahedral atom, most commonly carbon, bonded to four different groups A carbon with four different groups bonded to it is called a chiral center all chiral centers are stereocenters, but not all stereocenters are chiral centers Enantiomers: stereoisomers that are nonsuperposable mirror images Enantiomers: refers to the relationship between pairs of objects Smita Asthana @ St. Ann's 25

Optical Rotation and Polarimetry Smita Asthana @ St. Ann's 26

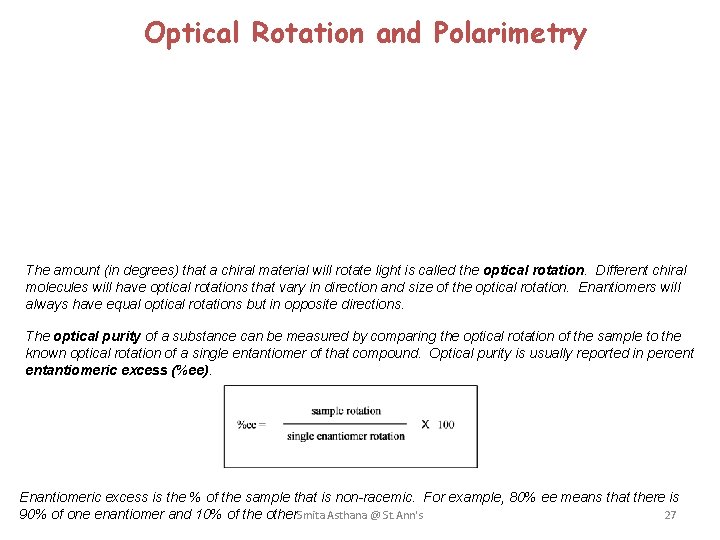
Optical Rotation and Polarimetry The amount (in degrees) that a chiral material will rotate light is called the optical rotation. Different chiral molecules will have optical rotations that vary in direction and size of the optical rotation. Enantiomers will always have equal optical rotations but in opposite directions. The optical purity of a substance can be measured by comparing the optical rotation of the sample to the known optical rotation of a single entantiomer of that compound. Optical purity is usually reported in percent entantiomeric excess (%ee). Enantiomeric excess is the % of the sample that is non-racemic. For example, 80% ee means that there is 90% of one enantiomer and 10% of the other. Smita Asthana @ St. Ann's 27

RACEMISATION i) By the action of heat ii) By treatment with chemical reagents iii) Autoracemisation RESOLUTION OF A RACEMIC MIXTURE The separation of a racemic mixture into its two enantiomers is called resolution. 1. Mechanical method – Physical Difference -Pasteur separated the crystals of sodium ammonium tartarate Na(NH 4) C 4 H 4 O 62 H 2 O (Racemate). Since this method brings experimental difficulties and has only limited application, it is only of historical importance now. 2. Biochemical method –Penicilium glaucum (a mould) when grown in a dilute solution of a racemate, it attacks the dextro from leaving behind the laevo form. Thus, the laevo form remains practically unaffected. Although, it is a slow method yet it is of wide application. Biochemcal method of separation has some disadvantages. i) One form is always destroyed ii) As dilute solutions re used, the amount of the second isomer left behind is very small. iii) It is difficult to select a micro-organism which attacks only one of the enantiomers Smita Asthana @ St. Ann's 28

3. Chemical method (Resolution by salt formation)- separation of racemic mixture of lactic acid. The racemic mixture of lactic acid is treated with 1 brucine ( a base) when salts, called diastereomers are obtained. dl lactic acid + I –brucine I-brucine-d-lactate+I-brucine I-lactate. The two lactates (diastereomers) are then separated by fractional crystallisation and then each is separately treated with HCI to get the two enantiomers of lactic acid. I-brucine-d-lactate+HCI d-lactic acid +I-brucine. HCI I-brucine-I-lactate+HCI I-lactic acid +I-brucine. HCI Smita Asthana @ St. Ann's 29

4. Kinetic method – Diffrence in rate of reaction - resolution of racemic mandelic acid; I-menthol (an alcohol) reacts faster with d-mandelic acid than with I-mandelic acid to form ester. Clearly, when, d. I-mandelic acid (a racemic mixture) is treated with limited quantity of I-menthol, the product formed is rich in d-ester then I-ester. 5. Selective adsorption- One of the enantiomers is selectively adsorbed on the surface of the adsorbent. The solution collected at the bottom of the adsorbent column is richer in the other enantiomer. This results in the separation of the racemic mixture. Smita Asthana @ St. Ann's 30

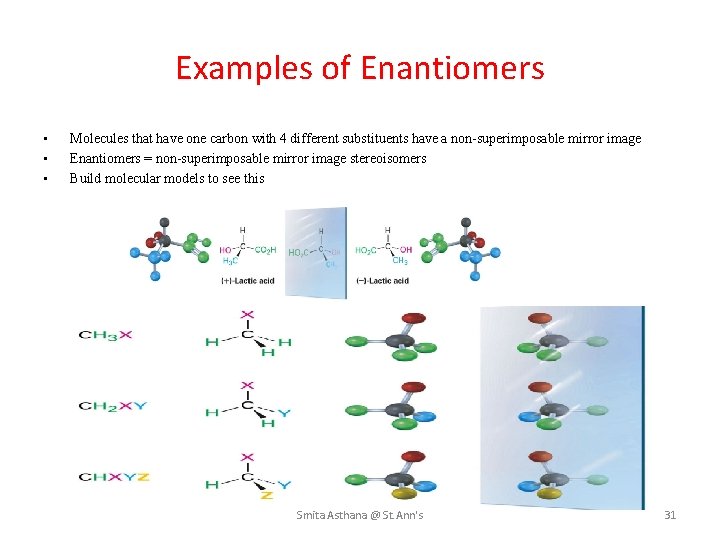
Examples of Enantiomers • • • Molecules that have one carbon with 4 different substituents have a non-superimposable mirror image Enantiomers = non-superimposable mirror image stereoisomers Build molecular models to see this Smita Asthana @ St. Ann's 31

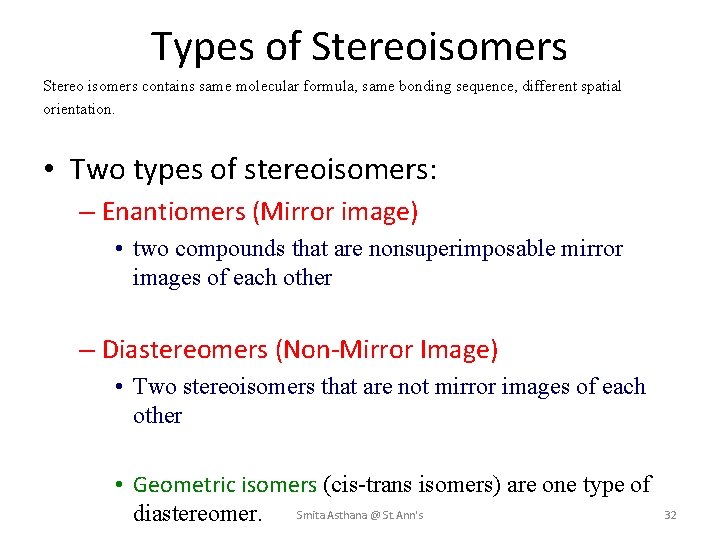
Types of Stereoisomers Stereo isomers contains same molecular formula, same bonding sequence, different spatial orientation. • Two types of stereoisomers: – Enantiomers (Mirror image) • two compounds that are nonsuperimposable mirror images of each other – Diastereomers (Non-Mirror Image) • Two stereoisomers that are not mirror images of each other • Geometric isomers (cis-trans isomers) are one type of diastereomer. Smita Asthana @ St. Ann's 32

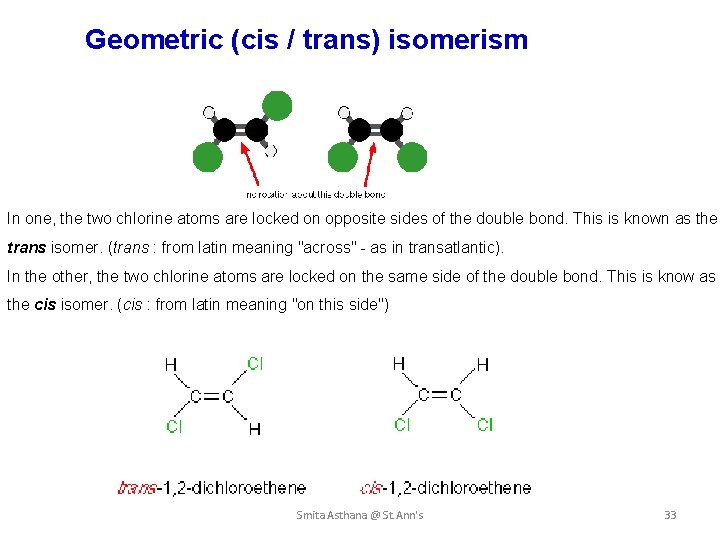
Geometric (cis / trans) isomerism In one, the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. (trans : from latin meaning "across" - as in transatlantic). In the other, the two chlorine atoms are locked on the same side of the double bond. This is know as the cis isomer. (cis : from latin meaning "on this side") Smita Asthana @ St. Ann's 33

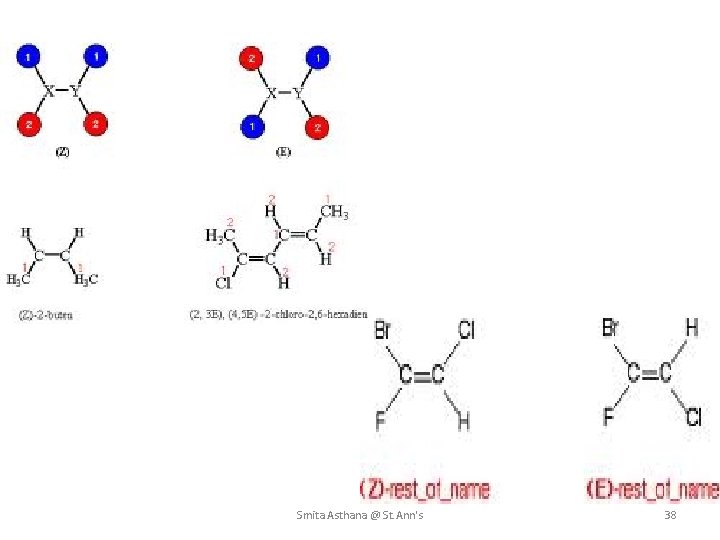
E-Z NOTATION The simple convention of denoting the geometrical isomers by cis/trans descriptors is not sufficient when there are more than two different substituents on a double bond. To differentiate the stereochemistry in them, a new system of nomenclature known as the E-Z notation method is to be adopted. According to this method, if the groups with higher priorities are present on the opposite sides of the double bond, that isomer is denoted by E. Where E = Entgegen ( the German word for 'opposite') However, if the groups with higher priorities are on the same side of the double bond, that isomer is denoted by Z. Where Z = Zusammen (the German word for 'together') The letters E and Z are represented within parentheses and are separated from the rest of the name with a hyphen. Smita Asthana @ St. Ann's 34

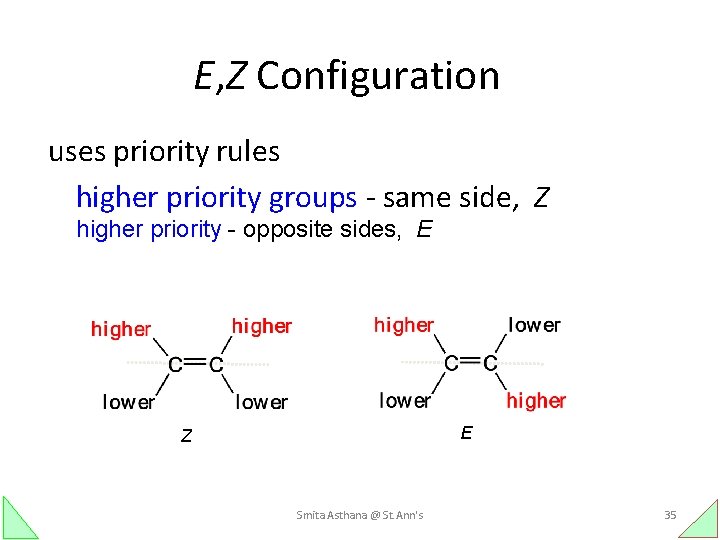
E, Z Configuration uses priority rules higher priority groups - same side, Z higher priority - opposite sides, E E Z Smita Asthana @ St. Ann's 35

Step by step procedure to determine the E-Z configuration The following procedure is to be adopted to denote the geometrical isomers by E & Z descriptors. 1. First determine the higher priority group on each end of the double bond. 2. If the higher priority groups are on the opposite sides of double bond, the isomer is denoted by the descriptor, E. 3. Otherwise if they are on the same side of double bond, the Z descriptor must be used. Smita Asthana @ St. Ann's 36

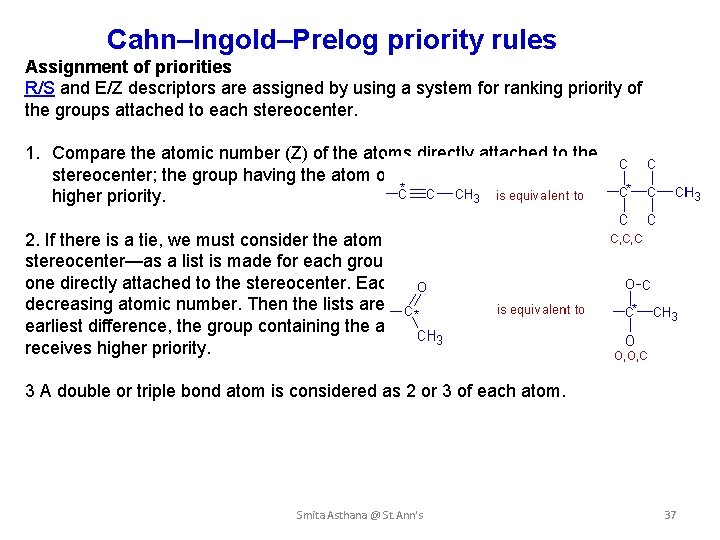
Cahn–Ingold–Prelog priority rules Assignment of priorities R/S and E/Z descriptors are assigned by using a system for ranking priority of the groups attached to each stereocenter. 1. Compare the atomic number (Z) of the atoms directly attached to the stereocenter; the group having the atom of higher atomic number receives higher priority. 2. If there is a tie, we must consider the atoms at distance 2 from the stereocenter—as a list is made for each group of the atoms bonded to the one directly attached to the stereocenter. Each list is arranged in order of decreasing atomic number. Then the lists are compared atom by atom; at the earliest difference, the group containing the atom of higher atomic number receives higher priority. 3 A double or triple bond atom is considered as 2 or 3 of each atom. Smita Asthana @ St. Ann's 37

Smita Asthana @ St. Ann's 38
 St anns college chirala
St anns college chirala Dr smita biswas
Dr smita biswas Smita mishra panda
Smita mishra panda Dr smita biswas
Dr smita biswas Peggy boylan-ashraf
Peggy boylan-ashraf Dr smita biswas
Dr smita biswas Mrs rajlaxmi is working
Mrs rajlaxmi is working Introduction to stereochemistry
Introduction to stereochemistry Finding stereocenters
Finding stereocenters Cyclopentane cis trans isomers
Cyclopentane cis trans isomers Naproxen stereochemistry
Naproxen stereochemistry Stereochemistry quiz
Stereochemistry quiz Stability of chair and boat conformation
Stability of chair and boat conformation Example of denatured protein
Example of denatured protein Father of stereochemistry
Father of stereochemistry Stereochemistry of cephalosporin
Stereochemistry of cephalosporin R vs s configuration
R vs s configuration